Summary
Questions remain to be answered about the use of radiation therapy in patients with breast cancer. So far, there is no way to identify patients who might not benefit from conventional or internal mammary node radiation therapy after breast-conserving surgery and adjuvant endocrine therapy.
- radiotherapy
- breast-conserving surgery
- partial breast irradiation
- internal mammary node radiotherapy
- brachytherapy
- PRIME II
- ISRCTN95889329
- RAPID
- NCT00282035
- ELIOT
- NCT01849133
- EORTC 22922/10925
- MA20
Radiotherapy (RT) after surgery has benefitted many patients; further research is needed to identify which patients could avoid RT after breast-conserving surgery (BCS) as well as the benefits of and how best to administer partial breast RT and internal mammary node radiotherapy (IMN-RT).
Ian H. Kunkler, MB BCHIR, University of Edinburgh, Edinburgh, United Kingdom, discussed randomized controlled clinical trials (RCTs) to determine which patients might avoid RT after BCS. In treating patients with low-risk breast cancer, an acceptable level of local recurrence and overall survival (OS) must be balanced with toxicity, quality of life, and the cost to the health care system. All of the RCTs evaluating RT after BCS have shown that RT significantly reduces local recurrence by two-thirds, and meta-analysis of the trials has shown that RT also improves survival [Early Breast Cancer Trialists’ Collaborative Group. Lancet. 2011]. The RCTs had varying eligibility criteria and differed in patient age, tumor size, tumor grade, use of tamoxifen, and attention to margins. To date, no studies have identified a subgroup of patients who could undergo BCS without RT [Jagsi R. CA Cancer J. 2014]. However, the absolute benefit of RT depends on the pretreatment risk of local recurrence. If patients could be identified who reliably have a very low risk of local recurrence, avoiding RT might place women at such a small risk of harm that they could safely be treated with BCS and no RT.
The PRIME II study [ISRCTN95889329; Kunkler IH et al. SABCS 2013 (abstr S2-01)] looked at the impact of omission of RT (n = 668) vs RT (n = 658) after BCS and endocrine therapy in low-risk older patients (mean age 71 years). The time to first local recurrence was significantly higher (P = .002) for no RT (4.1% 5-year actuarial rate for no RT vs 1.3% for RT). An unplanned subgroup analysis according to estrogen receptor (ER) status showed a benefit for RT regardless of ER status, although the benefit was greater for those with low ER. These results need to be confirmed. However, RT did not result in an OS benefit among patients treated in the PRIME II study who had overall a very low risk of local recurrence (4% at 5 years) even without RT.
Toxicities associated with RT include changes in breast appearance, shrinkage, induration, edema, and telangiectasia; reducing radiation dose might avoid some of these effects. Radiation-associated cardiac events are also RT dose dependent with no threshold [Darby SC et al. N Engl J Med. 2013]. RT is also associated with a risk of secondary malignancies, particularly lung and esophageal cancers, although data come from a meta-analysis of large cohort studies, not RCTs [Grantzau T, Overgaard J. Radiother Oncol. 2014].
Identification of biomarkers of low risk for ipsilateral breast tumor recurrence (IBTR) is needed to refine selection of low-risk patients who could avoid RT. Until then, the decision to omit RT must be made by individual patients and their health care providers, taking into account their stage and grade of disease, comorbidities, and expected life span.
Another RT strategy, discussed by Ivo A. Olivotto, MD, University of Calgary, Calgary, Alberta, Canada, is the use of partial breast irradiation. Accelerated partial breast irradiation (APBI) techniques were developed in the 1990s and early 2000s to deliver radiation to a smaller area of the breast in less time with a goal of reducing side effects and inconvenience. APBI techniques include brachytherapy, intraoperative RT, and 3D-conformal partial breast RT, which has been used the most, and is more accessible as a technique used for whole breast irradiation (WBI).
The RAPID study [NCT00282035; Olivotto IA et al. J Clin Oncol. 2013] looked at 3D conformal external beam partial breast irradiation (38.5 Gy/10 fractions BID; n = 1070) vs WBI (42.5 Gy/16 fractions or to 50 Gy/25 fractions; n = 1065). Adverse cosmesis (the main end point available at this time, assessed by trained nurses) was worse with APBI. At baseline, the proportion of women with fair or poor cosmesis was similar (17% to 19%) in both the WBI and APBI treatment groups; but at 3 years, adverse cosmesis was seen in 17% of WBI- vs 29% of APBI-treated patients. At 5 years, the rates of adverse cosmesis were 13% after WBI and 33% after APBI (P < .0001 for both time points) [Olivotto IA et al. J Clin Oncol. 2013]. These adverse effects on outcome with APBI were similar whether scored by nurses, physicians blinded to treatment, or the patients themselves.
Common toxicities that might be related to cosmetic outcome, including telangiectasia, induration/fibrosis, breast pain, and fatty necrosis were significantly more common in the APBI group at 3 years, and this could be related to the dose selected. With a median follow-up of just 3 years, there were too few local breast recurrences to assess efficacy.
A study of high dose-rate brachytherapy suggested similar rates of IBTR with APBI vs WBI, but the number of patients actually treated after difficulties caused by equipment or patient anatomy was low [Polgár C et al. Radiother Oncol. 2013].
In the ELIOT trial [NCT01849133], APBI with a single dose of intraoperative electrons after BCS (n = 651) resulted in a significantly higher rate of IBTR than whole breast external RT (n = 654) [Veronesi U et al. Lancet Oncol. 2013]. At a median 5.8 years of follow-up, the rates were 4.4% vs 0.4%, respectively (P = .0001). Patients treated with APBI with intraoperative low-energy photons alone in the TARGIT trial also had a higher risk of local recurrence compared to WBI [Vaidya JS et al. Lancet. 2014].
Brachytherapy merits further evaluation but APBI using the 3D conformal technique, dose, and fractionation in the RAPID trial should not be used. Prof Olivotto agreed with Prof Kunkler that it may be possible to identify patients with a very low risk of IBTR without RT. Trials using predictive markers in combination with patient age, tumor size, margin, and ER status are underway.
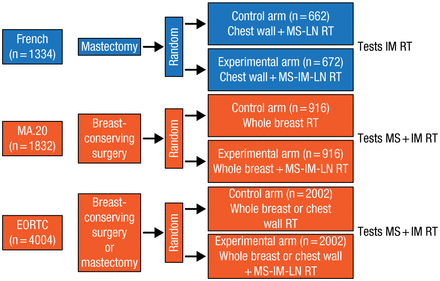
Another RT application, IMN-RT, was discussed by John R. Yarnold, MB BS, MRCP, Institute of Cancer Research and Royal Marsden National Health Service Foundation Trust, Sutton, United Kingdom. Prof Yarnold discussed 3 RCTs of IMN-RT. These trials, involving different patient populations and treatments, are summarized in Figure 1.
Treatment Comparisons in 3 Randomized Controlled Trials of Internal Mammary Node Radiotherapy
IM, internal mammary; LN, lymph node; MS, medial supraclavicular; RT, radiotherapy.
Adapted from Budach W et al. Adjuvant radiotherapy of regional lymph nodes in breast cancer - a meta-analysis of randomized trials. Radiat Oncol. 2013;8:267-274.
In the French trial [Hennequin C et al. Int J Radiat Oncol Biol Phys. 2013], which was conducted in the 1990s, OS at 10 years for IMN-RT was 62.6% vs 59.3% for no IMN-RT (P = .8). Disease-free survival (DFS) at 10 years for IMN-RT was 53.2% vs 49.9% for no IMN-RT (P = .35).
In the MA20 trial, there was no significant difference in OS (P = .07) between treatment groups. Distant DFS (metastasis-free survival) was significantly higher for WBI plus regional node irradiation (HR, 0.64; 95% CI, 0.47 to 0.85; P = .002) [Whelan TJ et al. J Clin Oncol. 2011 (abstr LBA1003)].
In the EORTC 22922/10925 trial, there was no difference in OS at 5 and 10 years between RT of the internal mammary (IM) and medial supraclavicular (MS) nodes vs no RT for IM-MS (HR, 0.87; 95% CI, 0.76 to 1.00; P = .056); there was an advantage for IM-MS RT for metastases-free survival (HR, 0.86; 95% CI, 0.76 to 0.98; P = .020) [Poortmans PM et al. ECC 2013 (abstr BA 2)].
Patient selection will be a major challenge in adopting IMN-RT as a standard of care, as it is not known which subgroups of patients would benefit, although Prof Olivotto believes that patients with positive axillary nodes or high-risk node-negative disease would benefit from IMN-RT. It is also not known if patients would benefit from IM node biopsy. Therefore, additional RCTs are needed to identify patients with breast cancer who would benefit from the various RT modalities available or in development.
- © 2014 SAGE Publications