Summary
This article highlights some important features of HER2-positive breast cancer. These include a review of some of the molecular mechanisms implicated in tumorigenesis, how certain therapies are used to interfere with these processes, the benefits of neoadjuvant therapy, and the treatment of patients with metastatic disease.
- metastatic disease
- HER2 signaling
- targeting
- resistance
- molecular mechanism
- trastuzumab
- lapatinib
- pertuzumab
- trastuzumab-emtansine
- CLEOPATRA
- VELVET
- EMILIA
- TH3RESA
In a session on HER2-positive breast cancer (BC), 3 presenters reviewed aspects of the disease and its management: basic mechanisms of HER2 signaling, targeting, and resistance; developments in neoadjuvant therapy; and the management of metastatic HER2-positive BC.
Mark M. Moasser, MD, University of California, San Francisco, San Francisco, California, USA, discussed HER2, part of the HER family, which comprises 4 transmembrane receptors: epidermal growth factor receptor (EGFR), HER2, HER3, and HER4. In pathologic states, loss of regulatory mechanisms permits massive HER2 overexpression, and HER3 plays an essential part in this process, which is not yet fully understood [Vaught DB et al. Cancer Res. 2012; Lee-Hoeflich ST et al. Cancer Res. 2008; Holbro T et al. Proc Natl Acad Sci USA. 2003].
There has been much interest in developing agents to target HER2 for the treatment of HER2-positive BC. Theoretically, inhibiting HER2 function is a reasonable strategy, and most studies have focused on the use of small-molecule inhibitors, such as lapatinib [Rusnak DW et al. Mol Cancer Ther. 2001], and antibodies, such as trastuzumab [Cho H-S et al. Nature. 2003]. However, this target has proven more difficult to inhibit than initially thought because of widespread and upfront resistance that is driven by a rapid compensatory increase in HER2-HER3 signaling output. However the strategy to home in on cancer cells via their massive surface HER2 expression using immunotherapy or immunodelivery approaches has proven far more impactful at this point with the introduction of trastuzumab, pertuzumab, and trastuzumab-emtansine introduced into clinical practice. Tumor PTEN and PIK3CA alterations have been extensively investigated but failed as potential predictive biomarkers of trastuzumab resistance (Loi S et al, J Natl Cancer Inst. 2013, Perez EA et al, J Clin Oncol. 2013, Pogue-Geile et al, J Clin Oncol. 2015). However biomarkers of immunologic signaling have proven powerful predictors of trastuzumab resistance (Perez EA et al, J Clin Oncol. 2015) and have identified an insufficient host immune response as a mechanistic basis for trastuzumab resistance. The development of more potent inhibitors of HER2-HER3 signaling is underway for more effective inactivation of oncogenic signaling, while the modulation of immune response may be a key next step in overcoming trastuzumab resistance.
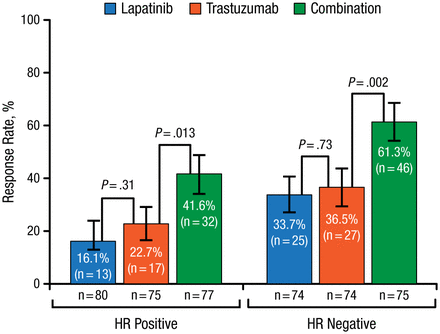
According to Sibylle Loibl, MD, PhD, German Breast Group, Neu-Isenburg, Germany, pathologic complete response (pCR) rate has increased in recent years from about 20% using chemotherapy alone, to almost 70% using long-term anthracycline-taxane chemotherapy regimens and double HER2 blockade. In a pooled analysis, the gap between pCR and no pCR was much larger in hormone receptor (HR)–negative compared with HR-positive patients [Cortazar P et al. Lancet. 2014]. In the NeoALTTO study [de Azambuja E et al. Lancet Oncol. 2014; Baselga J et al. Lancet. 2012], HER2 double-blockade using lapatinib and trastuzumab with taxane chemotherapy significantly increased both pCR (Figure 1) and survival rates.
Pathologic Complete Response by HR Status in NeoALTTO
HR, hormone receptor.
Reprinted from The Lancet, Copyright 2012;379:633-640, Baselga J et al, Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. With permission from Elsevier.
The phase 3 NOAH trial [Gianni L et al. Lancet Oncol. 2014; Gianni L et al. Lancet. 2010] demonstrated short- and long-term benefits of adding neoadjuvant trastuzumab to chemotherapy.
Predictive Biomarkers
PIK3CA (PIK3 catalytic subunit alpha) gene mutation occurs in about 20% of BC cases and is an independent predictor of pCR [Guarneri V et al. ESMO. 2014 (abstr 254O); Baselga J et al. ECC. 2013 (abstr 1859)]. Patients with PIK3CA mutation experience significantly lower pCR rates than those with wild-type PIK3 tumor [Loibl S et al. J Clin Oncol. 2014].
Prof Loibl showed data from the FinHER study, demonstrating that tumor-infiltrating lymphocytes predict trastuzumab benefit [Loi S et al. Ann Oncol. 2014].
Ian Krop, MD, PhD, Dana-Farber Cancer Institute, Boston, Massachusetts, USA, reviewed the treatment of metastatic HER2-positive BC.
First-Line Therapy
Pertuzumab is standard first-line therapy in patients with metastatic BC (MBC). The CLEOPATRA study [Baselga J et al. N Engl J Med. 2012] showed an overall survival benefit of almost 16 months in patients who received pertuzumab in addition to chemotherapy and trastuzumab, and the objective response rate was 80% [Swain S et al. ESMO. 2014 (abstr 350O)]. Toxicity with this combined antibody approach typically involves only mild increases in diarrhea and rash.
Docetaxel [Swain S et al. ESMO. 2014 (abstr 350O)] and paclitaxel [Datko F et al. SABCS. 2012 (abstr P5-18-20)] are acceptable chemotherapy partners to pertuzumab, said Dr Krop, and data from the VELVET study [Andersson M et al. ESMO. 2014 (abstr 361PD)] suggested that vinorelbine may also be suitable.
Dr Krop indicated that an off-label pertuzumab-based regimen may be reasonable in patients who missed out on first-line pertuzumab therapy [Baselga J et al. J Clin Oncol. 2010].
Second-Line Therapy
T-DM1 should be considered standard second-line therapy, said Dr Krop, since in the EMILIA study [Verma S et al. ESMO. 2012 (abstr LBA12)], T-DM1 demonstrated improved outcomes with favorable toxicity profiles, producing a median response of > 1 year. Randomized trials have also indicated its value as later-line therapy, including the TH3RESA study [Krop IE et al. Lancet Oncol. 2014] in which T-DM1 increased progression-free survival (6.2 vs 3.3 months; P < .0001), with a trend toward overall survival benefit.
Third and Later Lines of Therapy
In third-line and later therapy, combinations of trastuzumab or lapatinib and chemotherapy are active and should be continued, said Dr Krop, adding that while there are no preferred agents, he bases his choice on patient preferences.
Management of Central Nervous System Metastases
Central nervous system (CNS) disease occurs in up to 50% of patients with HER2-positive MBC. Radiation comprises the first-line therapy, and some patients benefit from resection. Single-agent lapatinib is of minimal benefit; this increases in combination with capecitabine, but duration of response is short [Sutherland S et al. Br J Cancer. 2010]. Although Dr Krop noted that the American Society of Clinical Oncology guidelines recommend treatment of MBC based on standard algorithms [Ramakrishna N et al. J Clin Oncol. 2014], he emphasized that improved therapies in this setting are needed. Clinical studies are underway to investigate systemic therapies, such as neratinib, that can cross the blood–brain barrier for the treatment of this challenging problem.
- © 2014 SAGE Publications