Summary
This article presents the 10-year results of a study comparing taxane type and schedule as a component of adjuvant chemotherapy in localized breast cancer. When administered after doxorubicin-cyclophosphamide, weekly paclitaxel and every-3-weeks docetaxel significantly improved outcomes in the entire study population, while weekly paclitaxel improved outcomes in triple-negative breast cancer.
- adjuvant therapy
- combination treatment
- axillary node positive
- disease-free survival
- doxorubicin
- cyclophosphamide
- paclitaxel
- A Phase 3 Study of Doxorubicin-Cyclophosphamide Followed by Paclitaxel or Docetaxel Given Weekly or Every 3 Weeks in Patients With Axillary Node-Positive Breast Cancer
- E1199
- BC
- NCT00004125
Joseph A. Sparano, MD, Montefiore Medical Center, Bronx, New York, USA, shared the 10-year results of the E1199 trial: A Phase 3 Study of Doxorubicin-Cyclophosphamide Followed by Paclitaxel or Docetaxel Given Weekly or Every 3 Weeks in Patients With Axillary Node-Positive Breast Cancer [BC; NCT00004125]. In the complete study population, adjuvant weekly paclitaxel and docetaxel Q3W significantly improved outcomes compared with paclitaxel Q3W, when administered sequentially after doxorubicin-cyclophosphamide (AC) therapy, and weekly paclitaxel improved outcomes in patients with triple-negative breast cancer (TNBC).
According to Dr Sparano, the E1199 trial was designed to compare taxane type and schedule as a component of adjuvant chemotherapy in localized BC [Sparano JA et al. N Engl J Med. 2008]. All participants received 4 cycles of standard AC therapy Q3W, and were randomized to 1 of 4 taxane arms: paclitaxel 175 mg/m2 Q3W for 4 doses (P3) or 80 mg/m2 weekly for 12 doses (P1); or docetaxel 100 mg/m2 Q3W for 4 doses (D3), or 35 mg/m2 weekly for 12 doses (D1). Patients received hormonal therapy according to standard of care. The primary end point was disease-free survival (DFS).
Dr Sparano reported that 1639 DFS events and 1283 deaths were recorded in 4950 patients at a median follow-up of 12.1 years. He added that primary comparisons showed no significant difference in outcomes with respect to taxane type (P1 + P3 vs D1 + D3; overall survival [OS], log-rank P = .977; DFS, log-rank P = .322) or schedule (P1 + D1 vs P3 + D3; OS, log-rank P = .795; DFS, log-rank P = .876). However, OS (P = .007) and DFS (P < .001) were significant when analyzed using a taxane-by-schedule interaction test, similar to reported 5-year outcomes.
With respect to secondary outcomes, there was a significant improvement in DFS (HR, 0.79; 95% CI, 0.68 to 0.90) and a trend toward increased OS (HR, 0.86; 95% CI, 0.73 to 1.00) with D3 compared with P3. Similar results were reported for DFS (HR, 0.84; 95% CI, 0.73 to 0.96) and OS (HR, 0.87; 95% CI, 0.75 to 1.02) for P1 compared with P3.
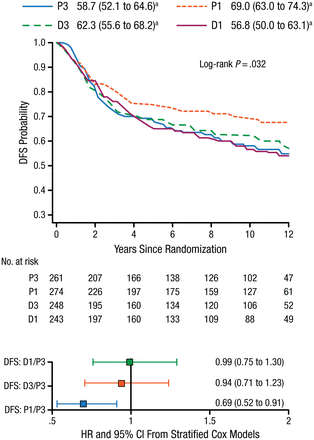
With respect to BC subtype, P1 was the most effective taxane regimen in patients with TNBC (n = 1025). This regimen was associated with increased OS (75% vs 66%; HR, 0.69, 95% CI, 0.50 to 0.94; P = .094) and DFS (69% vs 59%; HR, 0.69; 95% CI, 0.52 to 0.91; P = .032; Figure 1) compared with standard therapy (P3). Dr Sparano added that similar benefits were not seen with D3.
Improvement in DFS With Weekly Paclitaxel in Patients With TNBC
DFS, disease-free survival; D1, weekly docetaxel; D3, every-3-weeks docetaxel; P1, weekly paclitaxel; P3, every-3-weeks paclitaxel; TNBC, triple-negative breast cancer.
a10-y rate, % (95% CI).
Reproduced with permission from JA Sparano, MD.
For patients whose BC was hormone receptor–positive and human epidermal growth factor receptor-2–negative or of unknown status, Dr Sparano said that, although 5-year data showed a trend for improved outcomes in the taxane arms, this was not consistently seen in the updated analysis. At a median follow-up of 12.1 years in this patient population, there was a nonsignificant trend toward increased OS (81.6% vs 79.6%; HR, 0.87; 95% CI, 0.69 to 1.08) favoring D3 compared with P3. DFS was also improved in this group compared with P3 (75.3% vs 69.4%; HR, 0.76; 95% CI, 0.63 to 0.91). However, similar benefits were not seen in association with P1.
Although these data demonstrate benefit of AC and weekly paclitaxel therapy in patients with TNBC, Dr Sparano emphasized that outcomes can still be improved in this population. A pending trial [NRG-BR003] will investigate the effect of adding carboplatin to AC and weekly paclitaxel in this setting, he concluded.
- © 2014 SAGE Publications