Summary
Whether a biologic response modifier or triple disease-modifying antirheumatic drug (DMARD) therapy should be the mainstay of treatment for patients with rheumatoid arthritis (RA) is a subject for debate. This article compares triple conventional DMARD therapy versus biologic agents for the treatment of RA.
- Rheumatoid Arthritis
- Rheumatology
- Exclusive Article - For home page
- Rheumatoid Arthritis
Whether a biologic response modifier or triple disease-modifying antirheumatic drug (DMARD) therapy should be the mainstay of treatment for patients with rheumatoid arthritis (RA) is a subject for debate.
James R. O'Dell, MD, University of Nebraska Medical Center, Omaha, Nebraska, USA, presented the opinion that favors triple conventional DMARD therapy. In emphasizing a treat-to-target strategy, he said that conventional DMARD therapy should be started before biologics as initial treatment for RA, and conventional DMARDs should be combined with existing methotrexate (MTX) before biologics are added.
Dr. O'Dell argued that treatment for RA should hinge on value, the three components of which are efficacy, toxicity, and cost. He first discussed the efficacy component of value, pointing to major investigator-initiated studies in support of synthetic DMARDs. Dr. O'Dell supported his position using the TEAR study [Moreland LW et al. Arthritis Rheum 2012], in which there was no difference in the primary endpoint—the disease activity score in 28 joints (DAS28) plus erythrocyte sedimentation rate at Weeks 48 and 102—between patients randomized to triple therapy and those randomized to MTX plus etanercept.
Clinical outcomes between the two strategies as initial therapy were identical at 2 years in the BeST study [Goekoop-Ruiterman YP et al. Ann Intern Med 2007], at which time <15% of patients who were started on conventional DMARD therapy required step-up to a biologic. In an observational study conducted in two Nordic hospitals [Sokka T et al. Clin Exp Rheumatol 2013], remission/low disease activity was achieved at similar rates between therapy with combination conventional DMARDs and biologic drugs.
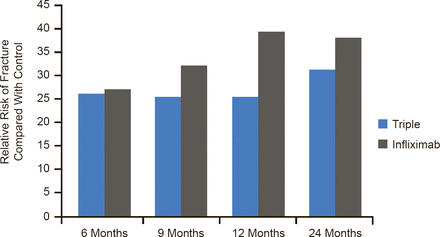
In examining the best strategy after MTX failure, Dr. O'Dell cited the RACAT study [O'Dell JR et al. N Engl J Med 2013], in which triple DMARD therapy was noninferior to etanercept plus MTX on the DAS28 endpoint in RA patients who had active disease despite MTX therapy. Twenty-four month data from the Swefot study [van Vollenhoven RF et al. Lancet 2012] also demonstrated no convincing clinical difference between adding DMARDs or a biologic after initial MTX failure, although 12-month data favored the biologic (Figure 1) [van Vollenhoven RF et al. Lancet 2009].
Swefot: Rates of Achieving Primary Outcome (EULAR Good Response) by Treatment
EULAR=European League Against Rheumatism; Swefot=Swedish Farmacotherapy.
Reproduced from van Vollenhoven RF et al. Addition of infliximab compared with addition of sulfasalazine and hydroxychloroquine to methotrexate in patients with early rheumatoid arthritis (Swefot trial): 1-year results of a randomised trial. Lancet 2009;374(9688):459–466. With permission from Elsevier.
If treatment is to target, the choice of agent does not matter, said Dr. O'Dell.
Ronald F. van Vollenhoven, MD, PhD, Karolinska Institute, Stockholm, Sweden, countered that biologic agents have revolutionized the treatment of RA and are clearly the more effective choice when used with MTX. Biologic agents are not only superior to conventional agents on the endpoint of disease activity, they act more quickly than conventional agents and are significantly superior to conventional agents on radiographic endpoints, he argued.
Finnish investigators, in their own investigator-initiated trial, Neo-RACo, found that the 2-year remission rate improved from 53% to 66% when patients with early active RA were treated with infliximab added to conventional intensified DMARDs compared with intensified treatment alone for the initial 6 months [Leirisalo-Repo M et al. Ann Rheum Dis 2013].
Addition of adalimumab to MTX and intra-articular steroids improved several endpoints including the DAS28-CRP, remission, function, and quality of life in the investigator-initiated OPERA study [Hørslev-Petersen K et al. Ann Rheum Dis 2013]. The additional improvement with adalimumab was ∼25% on these outcomes (Table 1).
OPERA: Outcomes by Treatment
In the Swefot study, the rate of good responses was better with the addition of infliximab than with the addition of sulfasalazine and hydroxychloroquine after MTX failure at both 12 months (39% vs 25%) [van Vollenhoven RF et al. Lancet 2009] and 24 months (43% vs 31%) [van Vollenhoven RF et al. Lancet 2012]. The difference at 24 months failed to achieve significance because the high number of dropouts left the study underpowered. Radiographic progression was significantly less in the biologic arm at 24 months, said Prof. van Vollenhoven.
Response is faster with biologics than with conventional DMARDs, he continued. RACAT showed a trend toward a more rapid response in patients assigned to etanercept-MTX compared with conventional combinations of DMARDs [O'Dell JR et al. N Engl J Med 2013]. It also demonstrated strong trends that favored etanercept in the change in DAS28 by 24 weeks, the percentage who achieved a DAS28 ≤3.2 and ≤2.6 (indicative of remission) at 24 weeks, American College of Rheumatology 50% improvement response criteria (ACR50) response at 24 weeks, and ACR70 response at 48 weeks, with a significant improvement (p=0.001) in the percentage who achieved an ACR70 response at 24 weeks. Radiographic outcomes were also superior with anti-tumor necrosis factor (TNF) therapy.
In his rebuttal, Dr. O'Dell said that although the TEAR trial did indeed show a faster response to biologics in patients with poor prognosis, patients on triple therapy did as well as those on biologics from Week 36 to Year 2 [Moreland LW et al. Arthritis Rheum 2012]. Similar results were obtained in the BeST trial of patients with early RA [Goekoop-Ruiterman YP et al. Ann Intern Med 2007]. In RACAT, patients were permitted to switch therapies with insufficient response to their original therapy, but by the end of the trial, outcomes were identical regardless of initial or secondary therapy [O'Dell JR et al. N Engl J Med 2013].
The small radiographic differences in favor of biologics, although statistically significant, are not clinically significant, said Dr. O'Dell. The advantage is typically in the range of one-half point on the 488-point total Sharp score (TSS). A 22-point difference would be required to detect a clinical difference on the TSS, he said.
In terms of toxicity, anti-TNF therapy was associated with double the rate of serious infections and triple the rate of malignancies compared with placebo in a meta-analysis [Bongartz T et al. JAMA 2006].
Biologics, as a class, are much better tolerated than conventional agents, said Prof. van Vollenhoven. He pointed to the higher rates of gastrointestinal and other complaints with conventional therapies [O'Dell JR et al. N Engl J Med 2013]. In RACAT, the withdrawal rate due to adverse events was more than double in patients assigned to conventional agents compared with patients assigned to biologics. Statistically significant higher rates of cardiovascular events and serious respiratory/thoracic/mediastinal events were also recorded among the patients assigned to conventional DMARDs.
Dr. O'Dell concluded with cost to support the position of triple DMARD therapy. In the Nordic hospitals study, despite equal outcomes, drug costs were nearly twice as high in the biologics group [Sokka T et al. Clin Exp Rheumatol 2013]. In TEAR, every quality-adjusted life-year gained in patients treated with biologics costs $837,100 [Jalal H et al. ACR 2013 (abstr 2646)].
While the economic considerations are legitimate, biologic therapy is the most effective treatment for RA today, said Prof. van Vollenhoven.
- © 2013 MD Conference Express®