Summary
This article presents the results from the Solitaire FR Thrombectomy for Acute Revascularisation trial [STAR; NCT01327989]. This was a prospective, observational, single-arm, multicenter, core lab-reviewed study conducted in high-volume stroke centers that had extensive mechanical thrombectomy experience. The Solitaire FR, a self-expanding retrievable stent, was utilized as a first-intention device in anterior circulation.
- Interventional Techniques & Devices Clinical Trials
- Interventional Techniques & Devices
- Neurology
- Neurology Clinical Trials
Vitor Mendes Pereira, MD, MSc, University Hospital of Geneva, Geneva, Switzerland, presented the results from the Solitaire FR Thrombectomy for Acute Revascularisation trial [STAR; NCT01327989]. This was a prospective, observational, single-arm, multicenter, core lab-reviewed study conducted in high-volume stroke centers that had extensive mechanical thrombectomy experience. The Solitaire FR, a self-expanding retrievable stent, was utilized as a first-intention device in anterior circulation.
The main inclusion criteria were patients aged 18 to 85 years with a National Institutes of Health Stroke Scale (NIHSS) score of 8 to 30, proximal anterior intracranial vasculature, and presentation within 8 hours of the onset of stroke, with a modified Rankin Scale (mRS) score ≤2 prior to stroke onset. Patients who were participating in another study, were pregnant or nursing, had signs of rapid neurological improvement, suffered a stroke within the previous 30 days, had an unknown time of stroke onset, or had a life expectancy <90 days were excluded. Additionally, any patients with evidence of intracranial hemorrhage, arteriovenous malformation, aneurysm, early ischemic changes, carotid dissection, complete carotid occlusions, vasculitis, or stenosis proximal to the thrombus site were excluded.
Of the 682 patients screened, 471 failed screening and 9 withdrew consent, for a total of 202 patients in the final study population. The median age was 72 years (range, 25 to 86), with 122 (60%) females and 80 (40%) males. The median NIHSS at baseline was 17 (range, 8 to 26). Stroke and procedure characteristics are presented in Table 1.
Stroke and Procedure Characteristics
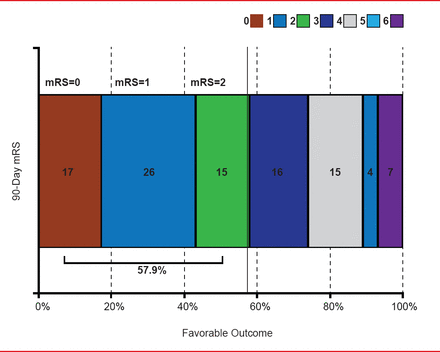
A total of 188 patients (93%) completed the 90-day follow-up and 14 (7%) died. Per the core lab, 160 patients (79%) met the primary endpoint of revascularization (thrombolysis in cerebral infarction score ≥2b) within 3 passes; 12 patients with missing endpoint data were counted as failures. Eighteen patients (9%) required rescue therapy, and 57.9% had a favorable outcome (mRS score ≤2) at 90 days (Figure 1). Per adjudication by Clinical Events Committee, 38 patients (18.8%) experienced symptomatic intracranial hemorrhage within 24 hours of the procedure. Fifteen patients (7.4%) had a device- or procedure-related serious adverse event.
Prof. Pereira said, “This nonrandomized prospective study suggests that treatment with the Solitaire FR device for intracranial anterior circulation strokes by comprehensive and experienced stroke centers results in a low risk of clinically relevant procedural and device related complications, high revascularization rates, and good clinical outcomes.” These results support the further investigation of this device in a randomized controlled trial against best medical treatment.
Modified Rankin Scale Distribution at 90 Days
mRS=modified Rankin Scale.
Reproduced with permission from VM Pereira, MD, MSc.
- © 2013 MD Conference Express®