Summary
Rechallenge of imatinib significantly improves progression-free survival and disease control rate in patients with advanced gastrointestinal stromal tumor (GIST) after the failure of at least imatinib and sunitinib, likely by continuous kinase inhibition of the bulk of disease clones which retain imatinib sensitivity. Tyrosine-kinase inhibitor (TKI)-resistant clones continue to progress, however, resulting in a relatively brief duration of benefit. This article presents data from the Rechallenge of Imatinib in GIST Having No Effective Treatment study [RIGHT; NCT01151852; Kang YK et al. J Clin Oncol 2013 (suppl; abstr LBA10502)], which evaluated the efficacy of imatinib rechallenge in patients with advanced GIST following failure of all TKIs.
- Gastrointestinal Cancers Clinical Trials
- Gastrointestinal Cancers
- Oncology
- Oncology Clinical Trials
Rechallenge of imatinib significantly improves progression-free survival (PFS) and disease control rate (DCR) in patients with advanced gastrointestinal stromal tumor (GIST) after the failure of at least imatinib and sunitinib, likely by continuous kinase inhibition of the bulk of disease clones which retain imatinib sensitivity. Tyrosine-kinase inhibitor (TKI)-resistant clones continue to progress, however, resulting in a relatively brief duration of benefit.
Despite having received highly effective treatments such as imatinib and sunitinib, >80% of patients with advanced GIST experience disease progression. Based on evidence of rapid GIST progression after discontinuation of all TKIs, common practice has been to resume imatinib therapy in these patients, even though the efficacy of this approach has not been proven in prospective clinical trials. Yoon-Koo Kang, MD, PhD, University of Ulsan College of Medicine, Seoul, South Korea, presented data from the Rechallenge of Imatinib in GIST Having No Effective Treatment study [RIGHT; NCT01151852; Kang YK et al. J Clin Oncol 2013 (suppl; abstr LBA10502)], which evaluated the efficacy of imatinib rechallenge in patients with advanced GIST following failure of all TKIs.
Eligible patients included adults with metastatic and/or unresectable GIST and prior benefit from first-line imatinib (defined as complete response [CR], partial response [PR], or stable disease [SD] for >6 months on imatinib 400 mg/day) and disease progression with at least both first-line imatinib and second-line sunitinib. Stratification was based on ECOG PS (0 to 1 vs 2 to 3) and use of third-line TKI. Subjects were randomized to receive oral imatinib 400 mg QD or placebo.
At the time of disease progression, subjects in the placebo group were permitted to cross over to open-label imatinib. Subjects receiving imatinib were permitted to continue or stop imatinib. The primary study endpoint was PFS determined by blinded external radiology review according to RECIST v1.0. Response was evaluated by computed tomography, every 4 weeks for the first 4 months then every 8 weeks until disease progression or death. Secondary endpoints included disease control rate (DCR: CR+PR+SD) at 12 weeks, overall survival (OS), time to progression, and safety.
Between July 2010 and January 2013, 81 patients were randomized (imatinib, n=41; placebo, n=40) at a single Korean center. More than 65% of the study participants were men; the median age was 60 years. Approximately 40% of subjects had received ≥3 prior TKIs. The small bowel was the most common disease site followed by the stomach. About 60% of patients had received imatinib as first-line therapy for >2 years.
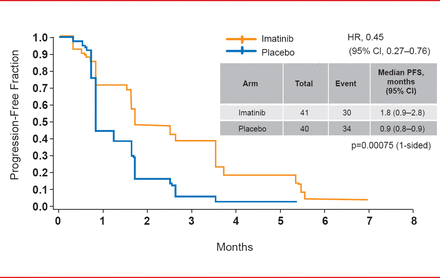
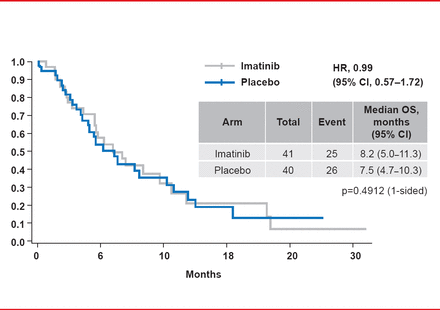
At study end in March 2013, median PFS was significantly longer for patients randomized to imatinib (1.8 months) versus placebo (0.9 months; HR, 0.45; 95% CI, 0.27 to 0.76; p=0.00075; Figure 1). The HR was <0.6 for all of the preplanned subgroups, strongly favoring imatinib. DCR at 12 weeks was 31.7% for imatinib versus 5% for placebo (p=0.003). The median PFS for the 37 subjects in the placebo arm who crossed over to imatinib after progression was 1.7 months, indicating the limited duration of the treatment response. Median OS was 8.2 months for imatinib versus 7.5 months for placebo (HR, 0.99; p=0.4912; Figure 2). The most common Grade 3 or higher treatment-emergent AEs during the double-blind period in the imatinib arm included anemia (29%), fatigue (10%), and hyperbilirubinemia (7%).
Progression-Free Survival
Reproduced with permission from YK Kang, MD, PhD.
Overall Survival
Reproduced with permission from YK Kang, MD, PhD.
- © 2013 MD Conference Express®