Summary
This article reviews data from four heart failure studies and discussed changes in the 2013 American College of Cardiology Foundation/American Heart Association Heart Failure Guidelines [Yancy CW et al. J Am Coll Cardiol 2013; Circulation 2013].
- Anemias
- Heart Failure
- Cardiology & Cardiovascular Medicine
Dipti Itchhaporia, MD, Hoag Heart and Vascular Institute, Newport Beach, California, USA, reviewed data from four heart failure studies and discussed changes in the 2013 American College of Cardiology Foundation (ACCF)/American Heart Association (AHA) Heart Failure Guidelines [Yancy CW et al. J Am Coll Cardiol 2013; Circulation 2013].
The primary change is the expanded definition of heart failure (HF) to HF with reduced ejection fraction (EF ≤40%; HFrEF or systolic HF) and heart failure with preserved ejection fraction (EF ≥50%; HFpEF or diastolic HF). Two subcategories of HFpEF have also been defined: HFpEF Borderline (EF 41% to 49%) and HFpEF Improved (patients previously diagnosed with HFrEF whose EF is now >40%).
Greater emphasis has been placed on adherence to performance and quality measures, reducing readmissions, patient self-care, and team-based care. The guidelines call for a more thorough analysis of HFpEF, a continued assessment of risk factors, genetic testing, and avoidance of anticoagulation in patients with chronic reduced EF and no risk factors. For the first time, the guidelines include recommendations for optimal guideline-directed medical therapy (GDMT).
RED-HF: ADDRESSING ANEMIA
Studies have shown that anemia in HF patients is associated with worse functional capacity and poor survival. Increasing hemoglobin using an erythropoiesis-stimulating agent has been suggested to have clinical benefit. Darbepoetin alfa is a glycoprotein that stimulates erythropoietin, a hormone released from the kidney that develops red blood cells and produces hemoglobin.
The Reduction of Events With Darbepoetin Alfa in Heart Failure study [RED-HF; Swedberg K et al. N Engl J Med 2013] assessed its effects on clinical outcomes in 2778 HF patients with HFrEF and mild-to-moderate anemia. Although darbepoetin alfa significantly increased hemoglobin levels, the increase did not reduce the risk of the primary composite outcome of death or hospitalization for HF. Scores on the Kansas City Cardiomyopathy Questionnaire indicated a small, but statistically significant improvement in quality of life (QoL; p=0.005) after 6 months in patients treated with darbepoetin alfa. The risk of thromboembolic events was significantly higher in darbepoetin alfa-treated patients (13.5% vs 10% with placebo). These findings suggested that hemoglobin is simply a marker of poor prognosis in HF rather than a therapeutic target.
ASTRONAUT: TESTING ALISKIREN, A NEW DIRECT RENIN INHIBITOR
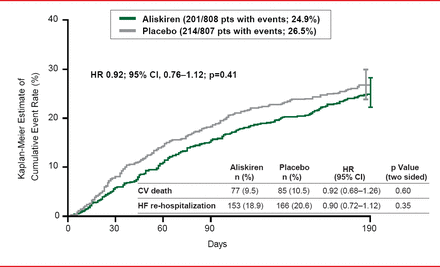
The ASTRONAUT trial [Gheorghiade M et al. JAMA 2013] was an international, randomized, controlled trial designed to evaluate the effect of inhospital initiation of the direct renin inhibitor aliskiren on postdischarge morbidity and mortality among patients with HF. The study population was comprised of patients hospitalized with HF who were hemodynamically stable with HFrEF, elevated natriuretic peptides (brain natriuretic peptide [BNP] ≥400 pg/mL or N-terminal pro-BNP [NT-proBNP] ≥1600 pg/mL), and signs and symptoms of fluid overload. Patients were recruited from 316 sites across North and South America, Europe, and Asia between May 2009 and December 2011. The follow-up period ended in July 2012. Patients were randomized to aliskiren (starting dose 150 mg, titrated to 300 mg as tolerated; n=808) or placebo (n=807), on top of standard therapy. The primary outcome of cardiovascular (CV) death or hospitalization for heart failure at 6 months occurred in 24.9% of the aliskiren group versus 26.5% of the placebo group (p=0.41).
The ASTRONAUT trial did not support routine administration of aliskiren in patients recently hospitalized for worsening chronic heart failure (Figure 1).
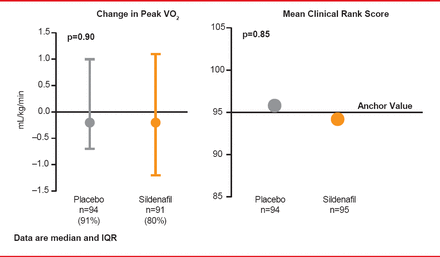
Data from observational studies and small trials have suggested that sildenafil, a phosphodiesterase-5 (PDE-5) inhibitor, might improve exercise capacity and clinical outcomes in HF patients compared with placebo. The Effect of Phosphodiesterase-5 Inhibition on Exercise Capacity and Clinical Status in Heart Failure With Preserved Ejection Fraction trial [RELAX; Redfield MM et al. JAMA 2013] was a randomized, controlled study that enrolled 216 patients with NYHA Class II to IV HFpEF at 26 North American centers between October 2008 through February 2012. Follow-up was through August 30, 2012. Participants were randomized to sildenafil (n=113) or placebo (n=103) administered orally at 20 mg TID for 12 weeks, followed by 60 mg TID for 12 weeks. The primary endpoint was change in peak oxygen consumption after 24 weeks of therapy. A key secondary endpoint was the composite clinical status rank score, based on time to death, time to CV or cardiorenal hospitalization, and change in QoL for participants without CV or cardiorenal hospitalization at 24 weeks.
CV Death or HF Rehospitalization Following Aliskiren Treatment
Reproduced from Gheorghiade M et al. Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: The ASTRONAUT randomized trial. JAMA 2013;309(11):1125–1135. With permission from the American Medical Association.
At Week 24 in RELAX, the change in peak oxygen consumption was not significantly different between the two groups, and there was no significant difference in mean clinical status rank scores (Figure 2).
Effects of Sildenfil on Exercise Capacity and Clinical Outcome
Reproduced from Redfield MM et al. Effect of Phosphodiesterase-5 Inhibition on Exercise Capacity and Clinical Status in Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial: Effect of PDE-5 on Exercise and Clinical Status in HFPEF. JAMA 2013;309(12):1268. With permission from the American Medical Association.
Even though HF remains a leading cause of hospital admission and readmission [Jencks SF et al. N Engl J Med 2009] the use of digoxin has been declining, in part due to its lack of effect on mortality and a downgrade in guideline recommendations. Older trials had shown benefit with digoxin, a drug discovered more than 2 centuries ago. The DIG trial [Digitalis Investigation Group. N Engl J Med 1997] showed that digoxin improved HF symptoms and reduced the risk of hospital admission both overall and for worsening heart failure. But, digoxin did not reduce overall mortality (Figure 4). In the RADIANCE trial [Packer M et al. N Engl J Med 1993] digoxin improved exercise tolerance and endurance, and patients switched from digoxin to placebo had lower QoL scores (p=0.04), decreased EF (p=0.001), and increases in heart rate (p=0.001) and body weight (p<0.001).
A recent post hoc analysis of the 3405 patients aged >65 years in the DIG trial showed that digoxin reduced the rate of all-cause hospital admission through 30 days (5.4% vs 8.1% with placebo) in ambulatory older patients with chronic HFrEF treated with ACE inhibitors and diuretics [Bourge RC et al. Am J Med 2013]. The absolute and relative risk for all-cause hospital admission were reduced by 2.7% and 34%, respectively, 30 days after randomization in patients randomized to digoxin. Digoxin reduced the risk of hospital admission due to CV causes by 47% (p<0.001) and worsening HF by 60% (p<0.001) during this same period.
These results are limited by their post hoc nature and other generalizability concerns. However, if they can be replicated in contemporary older HF patients discharged from the hospital after acute decompensation, digoxin may provide an inexpensive tool to reduce 30-day all-cause hospital readmission, the study authors stated.
Evidence-based, guideline-directed diagnosis, evaluation, and therapy should be the mainstay for all patients with HF, concluded Dr. Itchhaporia. Effective implementation of guideline-directed best quality care reduces mortality, improves QoL, and preserves healthcare resources. More research is needed to answer questions pertaining to prevention, nonpharmacologic therapy of HF (including dietary adjustments), treatment of HFpEF, management of hospitalized HF, effective reduction in HF readmissions, more precise use of device-based therapy, and cell-based regenerative therapy.
- © 2013 MD Conference Express®