Summary
This article discusses the results of the Durability of Combination Therapy With Exenatide/Metformin Versus Conventional Therapy in New Onset T2DM study [NCT01107717] that evaluated the effects of a triple-therapy regimen versus stepwise add-on conventional therapy in patients with newly diagnosed type 2 diabetes. In this open-label study, 155 patients were randomized to triple therapy or stepwise add-on conventional therapy, with a goal of achieving an HbA1C of <6.5%.
- Diabetes Mellitus
- Diabetes & Endocrinology Clinical Trials
- Diabetes Mellitus
- Diabetes & Endocrinology Clinical Trials
- Endocrinology
- Diabetes & Metabolic Syndrome
Muhammad Abdul-Ghani, MD, PhD, University of Texas Health Science Center, San Antonio, Texas, USA, presented the results of the Durability of Combination Therapy With Exenatide/Metformin Versus Conventional Therapy in New Onset T2DM study [NCT01107717] that evaluated the effects of a triple-therapy regimen versus stepwise add-on conventional therapy in patients with newly diagnosed type 2 diabetes. In this open-label study, 155 patients were randomized to triple therapy or stepwise add-on conventional therapy, with a goal of achieving an HbA1C of <6.5%. “We hypothesized that starting people with new-onset diabetes on agents that correct core defects known to be present in subjects with type 2 diabetes will produce a greater, more durable, and safer reduction in HbA1C,” explained Dr. Abdul-Ghani.
Triple therapy comprised metformin 1000 mg/day, pioglitazone 15 mg/day, and exenatide 5 μg BID. At Month 1, dosages were increased to metformin 2000 mg/day, pioglitazone 30 mg/day, and exenatide 10 μg/day. Pioglitazone was increased to 45 mg/day at Month 3 if glycemic targets (fasting plasma glucose [FPG] <100 mg/dL and HbA1C <6.5%) were unmet. Patients randomized to conventional therapy started with metformin 1000 mg/day. Medication adjustments were made at specific time points if glycemic targets (FPG <100 mg/day and HbA1C <6.5%) were not met: Month 1-metformin increased to 2000 mg/day and glyburide initiated at 5 mg/day; Month 2-glyburide increased to 10 mg/day; Month 3-glargine insulin initiated at 10 units/day. Glargine dosage was increased each week by 10 units/day if FPG remained >100 mg/dL.
Patients were followed every 3 months after the first 3 months. Changes could be made to medications if FPG was <60 mg/dL or in cases of hypoglycemia. Patients were considered treatment failures if they had HbA1C levels >6.5% on two consecutive visits 3 months apart despite maximum therapy. The primary endpoint was the difference in HbA1C between the triple-therapy and conventional-therapy groups.
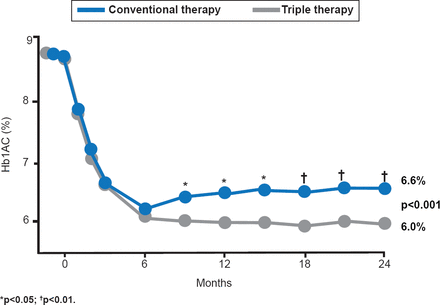
Participants were ∼47 years of age, with duration of diabetes of ∼5 months, and an initial mean HbA1C of 8.6% (range, 6.6% to 14.0%). HbA1C decreased sharply in both treatment groups by the 6-month time point. HbA1C remained below 6.5% for the triple-therapy arm from 6 to 24 months, but it gradually increased to 6.6% at 24 months in the conventional-therapy arm (Figure 1).
Change in HbA1C Over Time
Reproduced with permission from M Abdul-Ghani, MD, PhD.
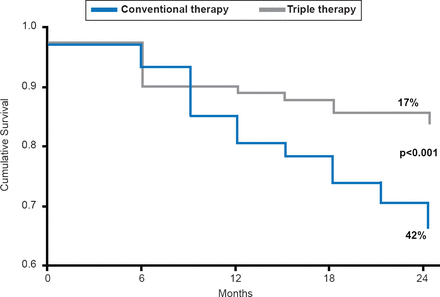
Additional analyses indicated that 60% of patients in the triple-therapy group achieved an HbA1C <6.0% versus 27% in the conventional-therapy group (p<0.001). At 24 months, significantly fewer patients in the triple-therapy group (17%) were considered treatment failures compared with the conventional-therapy group (42%; p<0.001; Figure 2).
Time to Treatment Failure
Reproduced with permission from M Abdul-Ghani, MD, PhD.
Patients in the triple-therapy group lost a mean of 1.2 kg whereas those in the conventional-therapy group gained a mean of 4.1 kg at 24 months (p<0.001). Significantly fewer hypoglycemic events occurred in the triple-therapy group (15%, 0.27 events per patient-year) versus the conventional-therapy group (46%, 2.1 events per patient-year; p<0.0001).
Dr. Abdul-Ghani concluded that initiating triple therapy with metformin/pioglitazone/exenatide at diagnosis achieves a greater and more durable reduction in HbA1C with less risk of hypoglycemia compared with the stepwise add-on conventional therapy.
- © 2013 MD Conference Express®