Summary
This article reports on the short-term findings of a randomized, double-blind, placebo-controlled crossover trial that demonstrated the prowess of the glucagon-like peptide-1 analogue liraglutide, used as an adjunct to insulin, in promoting glycemic control in type 1 diabetes [Heller SR et al. EASD 2013 (abstr 3); NCT01536665].
- Hyperglycemia/Hypoglycemia
- Diabetes Mellitus
- Diabetes & Endocrinology Clinical Trials
- Hyperglycemia/Hypoglycemia
- Endocrinology
- Diabetes & Metabolic Syndrome
- Diabetes Mellitus
- Diabetes & Endocrinology Clinical Trials
Simon Heller, University of Sheffield Medical School, Sheffield, United Kingdom, reported on the short-term findings of a randomized, double-blind, placebo-controlled crossover trial that demonstrated the prowess of the glucagon-like peptide-1 analogue liraglutide, used as an adjunct to insulin, in promoting glycemic control in type 1 diabetes (T1D) [Heller SR et al. EASD 2013 (abstr 3); NCT01536665].
The objective in this segment of the study, which is actually the secondary objective in the overall study, was to look at the safety and tolerability, as well as the pharmacodynamics of three different doses of liraglutide over 4 weeks of treatment. Liraglutide is not currently approved for treatment of T1D.
The study involved 45 adults with T1D. They were randomly allocated to receive a liraglutide dose of 0.6, 1.2, or 1.8 mg/day, administered initially as 0.6 mg/day with a weekly escalation of 0.6 mg until the target dose was reached. Each target dose was then maintained for at least 2 weeks. A fourth group received placebo. All groups were included as add-on to a 4-week regimen of insulin. A crossover design was used with a 2- to 3-week wash-out period.
Efficacy of the doses was ascertained by measuring daily insulin dose, change in HbA1C, and the mean of a 9-point self-measured plasma glucose (SMPG) determination. Safety was assessed by the number of episodes of hypoglycemia, number and type of adverse events (AEs), vital signs including pulse and blood pressure, and body weight.
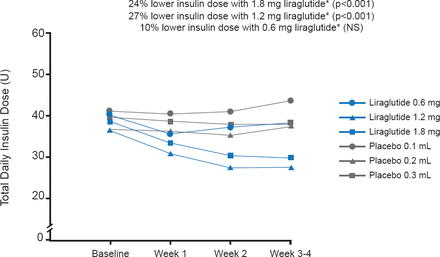
Similar baseline characteristics were evident for subjects in all four groups in terms of age (mean, 34.6 years), body mass index (∼23.9 kg/m2), HbA1C (∼7.6%), and duration of T1D (mean, 16.7 years). Also, the groups were similar in the daily insulin dose. At the end of the 4-week treatment, the liraglutide dose of 1.2 and 1.8 mg significantly lowered the daily insulin dose compared with placebo by 27% and 24%, respectively (p<0.001; Figure 1). The mean SMPG profile, change in HbA1C, and hypoglycemic events were similar in all groups (Table 1). Systolic and diastolic blood pressures did not differ appreciably. AEs were not serious, and the gastrointestinal maladies (mainly nausea, with some cases of diarrhea, vomiting, or other symptoms) observed more frequently with liraglutide use were expected. Of the five withdrawals, one was related to liraglutide-associated vomiting. The weight loss of up to 3.3 kg associated with liraglutide was not of concern, and could be beneficial.
Summary of Data
Reductions in Insulin Dose
*Compared with the corresponding placebo group.
Reproduced with permission from S Heller, MD.
The data support the use of liraglutide in glycemic control in T1D and pave the way for long-term, controlled trials.
- © 2013 MD Conference Express®