Summary
The optimal management of patients with atrial fibrillation (AF) is based on multiple patient-centered factors. Emerging research is demonstrating that a broad range of factors including biomarkers and genetics may help to further refine an individual patient's disease characteristics and may in turn help guide therapeutic decision making.
- Arrhythmias
- Cardiology Genomics
- Cardiology & Cardiovascular Medicine
The optimal management of patients with atrial fibrillation (AF) is based on multiple patient-centered factors. Emerging research is demonstrating that a broad range of factors including biomarkers and genetics may help to further refine an individual patient's disease characteristics and may in turn help guide therapeutic decision making.
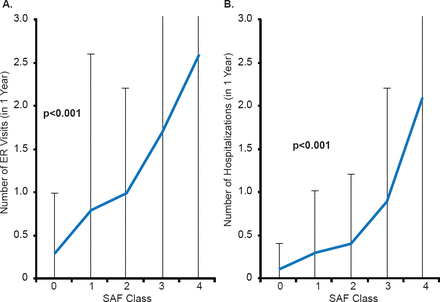
Ann M. Gillis, MD, University of Calgary, Calgary, Alberta, Canada, discussed the current recommendations for personalized medicine in AF based on clinical factors. The Canadian Cardiovascular Society (CCS) recommendations focus on decision-making based on a patient's individual balance of risk and symptom relief as well as quality of life. An important aspect to fulfilling this recommendation is assessing AF symptoms. One tool to objectively assess symptoms, the CCS Severity in Atrial Fibrillation (SAF) scale, includes both a mental and physical component [Dorian P et al. Circ Arrhythm Electrophysiol 2009]. As the SAF score increases from 0 to 4, the rate of emergency room presentation and hospitalization increase (p<0.001; Figure 1) [Angaran P, Dorian P. Can J Cardiol 2013]. AFNET and EHRA have proposed a very similar score, the EHRA score [Kirchhof P et al. Eur Heart J 2007], which has been used in the ESC guidelines on AF [Camm AJ et al. Eur Heart J 2010].
Severity in Atrial Fibrillation Score Associated With Healthcare Utilization
AFSS=atrial fibrillation severity scale; ER=emergency room; SAF=severity in atrial fibrillation.
Reproduced from Angaran P, Dorian P. Antiarrythmic Drugs in Atrial Fibrillation: Do They Have a Future? Can J Cardiol 2013; With permission from Elsevier.
Stanley Nattel, MD, Montreal Heart Institute, Montreal, Quebec, Canada, presented emerging targets in the personalized therapy of AF. Dr. Nattel noted that similar phenotypes of AF may actually have different molecular causes. For example, spontaneous ectopic activity in atrial cells appears to be triggered by ion current or ion transporter abnormalities, which result in delayed after depolarizations or “DADs” [Wakili R et al. J Clin Invest 2011]. A possible underlying mechanism for abnormal intracellular calcium ion levels is dysfunction of the ryanodine receptor (cardiac form is abbreviated “RyR2”), which causes abnormal “leak” of calcium into the cytosol during diastole [Voigt N et al. Circulation 2012]. In addition, up-regulation of the sodium-calcium exchanger (NCX) antiporter protein is associated with longstanding persistent AF and may contribute to DADs resulting from RyR2 calcium leak.
In paroxysmal AF, spontaneous ectopic activity in atrial cells appears to have similar underlying final endpoint mechanisms (ie, DADs) as in persistent AF. For example, calcium ion leak from RyR2 is increased [Voigt N et al. AHA 2013 (poster 9013)]. However, in paroxysmal AF, the sarcoendoplasmic reticulum calcium transport ATPase (SERCA) appears to be important, as its function is increased, resulting in sarcoplasmic-reticulum overload that increases the leakiness of RyR2s. One mechanism for increased SERCA activity is hyperphosphorylation of the SERCA-inhibitory protein phospholamban; phospholamban-hyperphosporylation causes phospholamban to dissociate from SECA, resulting in decreased SERCA-inhibition and enhancing the activity of SERCA. In addition, RyR2 function is aberrant in paroxysmal AF; however, it is not hyperphosphorylated. Unlike persistent AF, NCX expression and function are normal in paroxysmal AF.
Dr. Nattel highlighted that lessons learned from the differences in underlying mechanisms of persistent versus paroxysmal AF—the same phenotype may result from different molecular mechanisms; knowing the specific mechanisms in individual patients may be needed for successful therapeutic targeting.
Dr. Nattel discussed the idea that, in some patients, it may be possible to prevent structural remodeling, but early targeting may be needed. In an experimental system, inhibiting the renin-angiotensin system (RAAS) can prevent AF due to structural remodeling [Li D et al. Circulation 2001]. However, once tissue fibrosis, a key component of structural remodeling, has developed, therapy in experimental models, reversing the causative pathology may fail to reverse the fibrosis. Randomized, double-blind trials using RAAS inhibitors as secondary prevention have failed to show a benefit for secondary prevention in AF [Savelieva I et al. Europace 2011]. Perhaps a benefit would be observed if patients who are at risk but have not yet developed fibrosis were targeted.
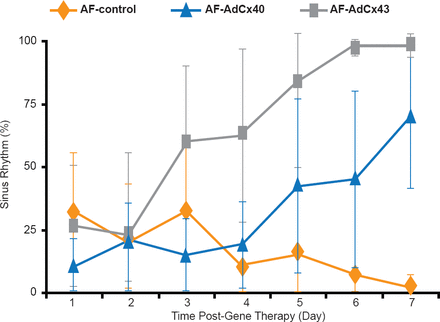
Dr. Nattel noted that it may be possible to specifically target molecular dysfunction, especially for defined genetic causes of AF. For example, connexin-gene abnormalities are one of the genetic causes of AF. A study in which animal models with AF received connexin gene-transfer via adenoviral vectors, the percentage of time spent in sinus rhythm was increased compared with control animals that did not receive connexin (Figure 2) [Igarashi T et al. Circulation 2011].
Effect of Connexin Gene-Transfer on Sinus Rhythm in Animal Models of AF
AdCx=connexin gene-transfer; AF=atrial fibrillation; SR=sinus rhythm.
Reproduced from Igarashi T et al. Connexin Gene Transfer Preserves Conduction Velocity and Prevents Atrial Fibrillation. Circulation 2012;125(2):216–225. With permission from Lippincott, Williams, and Wilkins.
John Camm, MD, St. George's University of London, London, United Kingdom, discussed proteomics as biomarkers for AF. The potential uses of biomarkers in AF include the prediction of incidence and prevalence of AF in various populations, the prediction of outcomes and treatment success, and the prediction of underlying mechanisms that guide treatment choice.
Inflammation as measured by the biomarker C-reactive protein (CRP) was demonstrated to significantly predict AF-free survival. In a prospective trial of 5806 patients, patients with CRP >1.92 mg/L experienced a lower AF-free survival rate compared with those with a CRP <1.92 mg/L (HR, 1.24; p<0.001) [Aviles RJ et al. Circulation 2003]. In addition, elevated CRP levels are associated with recurrence of AF following ablation [Jiang Z et al. Clin Cardiol 2013].
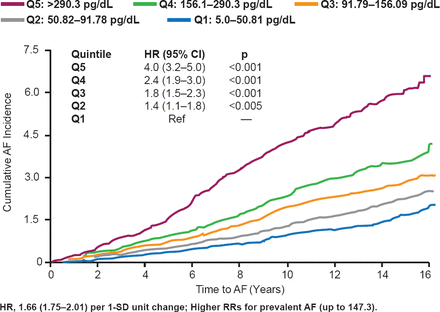
N-terminal prohormone brain natriuretic protein (NT-proBNP) levels are also associated with the development of AF. In a study of 5021 patients over a minimum of 10 years, patients with the highest quintile of NT-proBNP levels at >290.3 pg/dL were significantly more likely to develop AF than patients in any other quintile (HR, 4.0; 95% CI, 3.2 to 5.0; p<0.001; Figure 3) [Patton KK et al. Circulation 2009]. And, as NT-proBNP quartile levels increased, the cumulative incidence of AF also increased. In addition, in the ARISTOTLE trial, apixaban demonstrated greater efficacy in patients with greater NT-proBNP levels over 30 months [Hijazi Z et al. J Am Coll Cardiol 2013]. In a study of 584 patients with ischemic stroke, 38% of patients with a BNP of >400 pg/mL had AF, 26% with BNP levels of 200 to 400 pg/mL, and 12% of those with a BNP level of 100 to 200 pg/mL [Shibazaki K et al. Am J Cardiol 2012].
Association of NT-proBNP Levels and Development of AF
AF=atrial fibrillation; Q=quintile.
Reproduced from Patton KK et al. N-Terminal Pro-B-Natriuretic Peptide is a Major Predictor of the Development of Atrial Fibrillation. The Cardiovascular Health Study. Circulation 2009. With permission from Lippincott, Williams and Wilkins.
Other potential biomarkers include troponin I, matrix metallopeptidase 9 (MMP-9). In plasma samples from 6189 patients with AF in the RE-LY trial, increased troponin I levels were associated with an increase in cumulative events [Hijazi Z et al. Circulation 2012]. In the Atherosclerosis Risk in Communities [ARIC] cohort of 13718 without AF during the early 1990s, MMP levels were assessed in a random sample of 500 patients without AF and 580 patients that had developed AF during the follow-up time of ∼12 years [Huxley RR et al. PLoS One 2013]. Patients that developed AF were more likely to have elevated MMP-9 levels than patients without AF (HR, 1.27; 95% CI, 1.07 to 1.50; p=0.006) compared with other markers, such as MMP-1, MMP-2, TIMP-1, TIMP-2, and CICP. In addition, higher concentration of MMP-9 is associated with recurrence of AF following cardioversion (p<0.01) [Mukherjee R et al. J Cardiovasc Trans Res 2013].
Patrick T. Ellinor, MD, PhD, Harvard Medical School, Boston, Massachusetts, USA, discussed genetic risk markers of AF. According to data from the Framingham Heart Study, strong clinical risk factors for the development of AF include congestive heart failure and valve dysfunction, followed by age, diabetes, hypertension, and myocardial infarction. In addition, the presence of AF in a first-degree relative is associated with an increased risk of AF (HR, 1.39; 95% CI, 1.12 to 1.73; p=0.003) [Lubitz SA et al. JAMA 2010].
In 2012, a genome-wide association study (GWAS) of 16 studies that included 6624 patients with AF and 52426 patients without AF identified 9 genetic loci for AF [Ellinor PT et al. Nat Genet 2012; Ellinor PT et al. Nat Genet 2010; Benjamin EJ et al. Nat Genet 2009; Gudbjartsson DF et al. Nat Genet 2009; Gudbjartsson DF et al. Nature 2007]. The loci identified included KCNN3, PRRX1, PITX2, CAV1, C9ORF3, MYOZ1, SYNE2, HCN4, and ZFHX3. Multiple genetic signals have been identified around PITX2, a gene that is associated with developing AF. In addition, a recent GWAS found that the top two genetic loci for AF, PITX2 and ZFHX3, were also strongly associated with cardioembolic stroke [Bellenguez C et al. Nat Genet 2012]. Interestingly, PITX2 is expressed only in the left atrium [Kirchhof P et al. Circ Cardiovasc Genet 2011].
Paulus Kirchhof, MD, University of Birmingham, Birmingham, United Kingdom, discussed the potential personalization of rhythm control therapy in patients with AF with a focus on imaging and electrocardiogram (ECG) markers.
Imaging markers may provide individualized data that may improve outcomes. For example, focal impulse and rotor modulation ablation guided by ECG resulted in a significant increase in event-free survival in patients with AF (p<0.016) [Narayan S et al. J Am Coll Cardiol 2012]. In a study of 635 patients with persistent AF, ECG and response to flecainide that guided cardioversion resulted in a survival probability of 86% at 6 months [Kirchhof P et al. Lancet 2012 (subanalysis pending publication)].
Prof. Kirchhof pointed out that the management of patients with AF is already personalized as it is dependent on clinical information. However, improvement is needed in treatment that targets individual AF-causing mechanisms, which may reduce the high rates of complications of AF therapy.
In conclusion, the optimal treatment of AF is dependent on the severity of AF and comorbid conditions, such as the presence of hypertension, coronary artery disease, and heart failure. Personalized management of AF is becoming increasingly refined and in the future is likely to include a broad range of factors beyond clinical characteristics such as evidence of atrial remodeling, measurement of atrial stress/strain, biomarker concentrations, genomics, ECG patterns and AF patterns, state of the disease, and biologic age and well-being [Gillis AM et al. Can J Cardiol 2013].
- © 2013 MD Conference Express®