Summary
The Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Arterial Stenosis trial [SAMMPRIS; NCT00576693] found that treatment with aggressive medical management (AMM) was superior to AMM plus percutaneous transluminal angioplasty and stenting [Chimowitz MI et al. N Engl J Med 2011]. This article presents a detailed analysis of 30-day outcomes from the stenting arm of the SAMMPRIS trial.
- Neurology Clinical Trials
- Cerebrovascular Disease
- Interventional Techniques & Devices
- Thrombotic Disorders
- Interventional Radiology
Symptomatic intracranial stenoses are an important cause of stroke, which has a high risk of recurrence with medical therapy [Chimowitz MI et al. N Engl J Med 2005]. Yet, the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Arterial Stenosis trial [SAMMPRIS; NCT00576693] found that treatment with aggressive medical management (AMM) was superior to AMM plus percutaneous transluminal angioplasty and stenting (PTAS) [Chimowitz MI et al. N Engl J Med 2011]. Colin Derdeyn, MD, FAHA, Washington University School of Medicine, St. Louis, Missouri, USA, presented a detailed analysis of 30-day outcomes from the stenting arm of the SAMMPRIS trial.
The number of patients who underwent angioplasty/stenting was 213. Post hoc analyses were all bivariate, without Bonferroni correction for multiple comparisons, and event rates were small. A total of 60 variables were used in the analysis.
At 30 days, there were 7 parenchymal brain hemorrhages, one of which was asymptomatic, and 6 subarachnoid hemorrhages (SAH), one of which was asymptomatic; the others were caused by wire perforation or vessel rupture.
Postprocedure timing of intraparenchymal hemorrhage (IPH; n=7) ranged from immediate to 3 days. One case was symptomatic immediately postprocedure; 4 occurred within 4 to 24 hours; one occurred 2 days after the procedure; and another occurred at 3 days. All were distributed within the vascular territory of the targeted artery and were most likely secondary to reperfusion.
The interval between the qualifying event and PTAS ranged from 3 to 32 days. Outcomes were typically severe; 4 were fatal; one had an mRS score of 5 and another had 2; and one was asymptomatic. Significant and select factors for IPH included baseline percent of stenosis (central; p=0.011); preangiography diameter stenosis (DS; mm; p=0.01); JPEG review (p=0.042); and preangiography DS (mm; p=0.058). This suggests a common theme of small vessel diameter as a risk factor for IPH after stenting.
Six patients had periprocedural subarachnoid hemorrhages. All were recognized during or immediately after the procedure. Three were most likely guidewire perforations or vessel rupture. Obvious perforation was controlled with coil or glue occlusion of the vessel (n=2).
Two suspected cases of occult perforation were confirmed with CT imaging during or immediately after the procedure; one was asymptomatic, and the other was related to wire perforation.
Significant factors for wire perforation included stent diameter (p=0.051), preangiography percent stenosis (local; p=0.012), and max balloon inflation time (sec; p=0.005). These factors suggest that small vessel diameter predisposes one to SAH after stenting.
Ischemic complications in treated lesions included 12 local perforator distributions, 5 embolic strokes, and 2 delayed stent occlusions that occurred at 4 and 6 days. Both complications resulted in large ipsilateral strokes.
Significant and other select factors for ischemic strokes (n=19) included having diabetes (p=0.017), mean age (p=0.024), and symptomatic artery (p=0.059). For procedural perforator strokes, significant factors included lesion length (p=0.075), mean age (p=0.072), and symptomatic artery (p=0.017). For procedural embolic strokes (n=5), they included serum glucose (p=0.058), baseline stenosis percentage (local; p=0.096), and baseline Dn (local) mm (p=0.076). Most procedure-related ischemic strokes were local perforator territory and most were in the basilar artery.
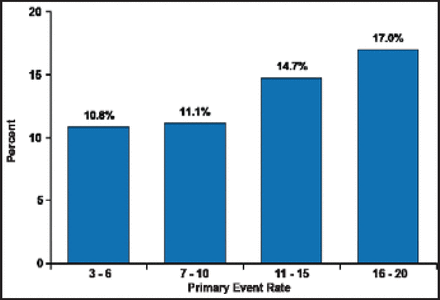
Periprocedural primary event rate as a function of wingspan volume is shown in Figure 1. Thirty-day primary endpoint rates at sites with the most experience in registries and in SAMMPRIS are shown in Table 1. Interventionists that came into the trial with less wingspan experience did not have higher rates of 30-day primary endpoints than those with more experience. These data suggest that the credentialing process selects physicians with good technical expertise.
Thirty-Day Primary Endpoint Rates at Sites With The Most Experience in Registries and in SAMMPRIS.
Operator Wingspan Credentialing Volume.
Reproduced with permission from C. Derdeyn, MD.
Dr. Derdeyn concluded that angioplasty and stenting in a SAMMPRIS-eligible population carries high risk, owing to increased hemorrhage risk in smaller diameter vessels and increased ischemic stroke risk in perforator-rich vessel segments. Unfortunately, most symptomatic intracranial stenoses involve either small-diameter vessels or perforator-rich vessel segments. The relative lack of experience with the wingspan device was not a factor in the outcomes in the stenting arm of the trial.
- © 2012 MD Conference Express®