Summary
According to the results from the Randomized Trial to Assess Efficacy of PUFA for the Maintenance of Sinus Rhythm in Persistent Atrial Fibrillation [FORWARD; NCT00597220], pharmacological supplementation with n-3 polyunsaturated fatty acids (PUFA) does not reduce recurrent atrial fibrillation.
- Arrhythmias
- Cardiology Clinical Trials
According to the results from the Randomized Trial to Assess Efficacy of PUFA for the Maintenance of Sinus Rhythm in Persistent Atrial Fibrillation [FORWARD; NCT00597220] presented by Alejandro Macchia, MD, Fundación GESICA, Buenos Aires, Argentina, pharmacological supplementation with n-3 polyunsaturated fatty acids (PUFA) does not reduce recurrent atrial fibrillation (AF).
Results from previous epidemiological studies and small clinical trials have been inconclusive regarding the ability of PUFA to reduce or prevent AF. The objective of this study was to test the efficacy of pharmacologic supplementation (1 g/day for 1 year) of n-3 PUFA for the maintenance of normal sinus rhythm in patients with previous AF.
The patient population consisted of men and women at least 21 years of age who had recovered normal sinus rhythm after having been diagnosed in an outpatient setting with symptomatic AF. Patients had either paroxysmal AF (defined as at least 2 symptomatic episodes of documented AF in the previous 6 months before randomization with the last episode occurring within 3 to 90 days before enrolling) or persistent AF (defined as successful electrical or pharmacological cardioversion performed within 3 to 90 days before study enrollment). Patients with lone AF, class IV congestive heart failure (CHF), acute coronary syndrome (ACS), or cardiac surgery in the previous 3 months were not eligible for the study. The presence of significant valvular disease, Wolff-Parkinson-White syndrome, planned or recent implantation of a cardiac device, ablative treatment for AF, or any arrhythmia associated with an acute reversible condition were also cause for exclusion.
The primary efficacy endpoint was the time to first recurrence of symptomatic or asymptomatic AF as documented by a 12-lead ECG. Secondary endpoints included the hierarchical composite of all-cause mortality, nonfatal stroke, nonfatal acute myocardial infarction, systemic embolism, CHF development, and severe bleeding; all-cause hospitalization; survival free of thromboembolic events; and hospitalization for cardiovascular reasons. Follow-up clinical visits occurred at 2, 4, 8, and 12 months.
A total of 586 patients received either n-3 PUFA (n=289) or a placebo (n=297). Subjects were mean 66 years of age. Approximately 73% of subjects were enrolled because of single episode of AF that required electrical cardioversion, 63% received amiodarone, 61% received a β-adrenergic receptor blocking drug, and more than 90% were hypertensive.
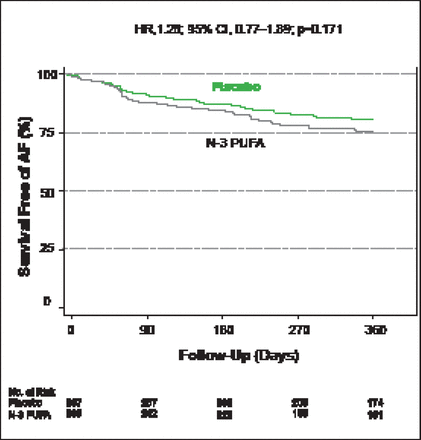
After 1 year of follow-up there was no significant difference in the primary efficacy endpoint of survival free of AF between the n-3 PUFA- and placebo-treated patients (HR, 1.28; 95% CI, 0.77 to 1.89; p=0.171; Figure 1), nor were there any significant differences regarding the other prespecified endpoints.
Survival Free of AF.
Reproduced with permission from A Macchia, MD.
According to Dr. Macchia, when the results of this study are added to those of previous studies with a similar hypothesis, the results are clearly neutral, leading to the conclusion that there is no role for n-3 PUFA in the secondary prevention of AF.
Future cardiovascular society guideline updates for the treatment of AF will likely incorporate these findings and note the lack of any benefit for preventing AF recurrence with the tested PUFA regimen and patient population. The benefits (or lack thereof) of other PUFA strategies, including natural sources from fish consumption or use in other patient populations such as lone or new-onset AF, remain unknown.
- © 2012 MD Conference Express®