Summary
The following is a summary of presentations from a session that focused on potential improvements in imaging in the design of clinical trials in stroke.
- Neurology
- Neuroimaging
- Neuroimaging Clinical Trials
The following is a summary of presentations from a session that focused on potential improvements in imaging in the design of clinical trials in stroke.
Patient Selection With PWI/DWI
Perfusion- and diffusion-weighted MRI (PWI/DWI) are imaging technologies that are used increasingly in acute stroke intervention trials to differentiate salvageable from irreversibly injured tissue. Gregory W. Albers, MD, Stanford University Medical Center, Palo Alto, California, and winner of the 2011 Sherman Award, discussed using PWI/DWI for patient selection in acute stroke reperfusion trials beyond the 3-hour window. The key to the selection process is enrolling patients who are most likely to benefit from reperfusion and unlikely to be harmed. These patients have small, irreversibly injured cores, and large regions with salvageable critical hypoperfusion. Although access to urgent MRI scanning is challenging compared with computed tomography (CT), it is more accurate for identifying the volume and location of the irreversibly injured infarct core, a critical factor in patient selection.
The use of DWI to define irreversibly damaged infarct core has been challenged by data that suggest the potential for partial reversal of early DWI lesions; however, recent data from the EPITHET and DEFUSE studies demonstrate that true DWI lesion reversal is uncommon in ischemic stroke patients and that when reversal does occur, the volume is very small [Chemmanam T et al. Neurology 2010]. In addition, although transient reversal of DWI lesions following early reperfusion is common, animal studies indicate that transient reversal is associated with widespread neuronal necrosis [Li F et al. Stroke 2000]. Recent studies have confirmed the importance of establishing the size of the early ischemic core; patients who present with a large volume of irreversibly injured tissue (>70–100 cc) do not respond favorably to reperfusion therapy [Albers et al. An Neurol 2006; Yoo AJ et al. Stroke 2009].
Recent data, including a study in which PET scans were performed shortly after PWI scans in acute stroke patients, demonstrate that PWI, using a threshold of Tmax >5.5, can identify penumbral tissue with high sensitivity and specificity [Zaro-Weber O et al. Stroke 2010].
Dr. Albers concluded that accurate identification of core and penumbra is the fundamental challenge to patient selection for extended window clinical trials. “MR can deliver on both of these critical issues, and we should do more to increase our access to acute MRI scans.”
Using Dynamic CT to Assess Perfusion
Dynamic CT perfusion imaging reliably detects tissue that is at risk in cases of acute stroke and is a feasible method for any clinic with a third-generation CT scanner (16-slice or greater). Mark Parsons, MD, PhD, University of Newcastle, New South Wales, Australia, discussed the advantages of using CT perfusion in acute stroke intervention trials.
Advanced imaging tools are crucial to rescuing salvageable tissue, the “holy grail” of acute stroke treatment and future interventional trials. Rapidity of access to MRI is a significant issue, as screening for safety in hyperacute situations can lead to delays, and in some countries, MRI is often monopolized for less urgent outpatient indications.
Perfusion computed tomography (PCT) shows promise in acute stroke assessment. PCT is readily accessible in most clinical centers. Maps of time to peak (TTP), Tmax, cerebral blood volume (CBV), and cerebral blood flow (CBF) can be calculated from the dynamically enhanced scans. CBF maps are excellent predictors of the extent of infarct core [Mayer TE et al. Am J Neuroradiol 2000; Bivard et al. Cerebrovascular Diseases 2011].
Noncontrast CT and CT with angiography source images underestimate the baseline infarct core size relative to CTP. In patients who have CTP within 6 hours of stroke, assessment of collateral status using combined CTA and CTP was able to identify core infarct regions, predict the fate of penumbral tissue, and increase the chance of more favorable functional outcomes (modified Rankin Scale score 0–2) [Miteff F et al. Brain 2009].
In conclusion, Prof. Parsons noted, perfusion CT is a good tool for assessing core and penumbra tissue regions and is crucial for future acute stroke interventional trials.
Thrombus Detection Using CTA
Trials, such as European Cooperative Acute Stroke Study (ECASS) and National Institute of Neurological Disorders and Stroke (NINDS II), have shown only a 7% effect size for IV thrombolytics in the treatment of acute ischemic stroke. Rüdiger Von Kummer, MD, PhD, Dresden University Stroke Center, Dresden, Germany, discussed why the majority of patients fail to benefit from IV tPA treatment and how that might be changed.
The primary reasons for lack of benefit from tPA in some patients are recanalization/reperfusion failures (eg, not achieving recanalization/reperfusion, achieving recanalization but only partial reperfusion) and reocclusion in addition to patients with spontaneous reperfusion at admission. Some of the more important conditions that might influence arterial patency include tandem arterial obstruction internal carotid artery/middle cerebral artery (MCA) and having large thrombi or tight atherosclerotic stenosis. The relative resistance to thrombolysis in tandem lesions varies, depending on the location of the MCA clot, an outcome that may be improved with more informed patient selection [Rubiera M et al. Stroke 2006]. In a study of proximal vessel occlusions, baseline CTA identified those patients who were likely to have a low rate of recanalization following IV tPA [Bhatia R et al. Stroke 2010]. It also might be possible to quantify the extent of vascular obliteration more accurately with thin-slice non-enhanced CT reconstructions [Riedel CH et al. Stroke 2010]. Further early arterial reocclusion on transcranial Doppler is highly predictive of clinical deterioration and poor long-term outcome [Saqqur M et al. Stroke 2007].
Prof. von Kummer concluded that “assessment of arterial obstruction is mandatory before and after treatment in order to identify patients not likely to benefit from IV rtPA.”
A Simplified Vascular Imaging Paradigm
“The problem we face in stroke is we have had too many failed pivotal acute stroke trials,” said Andrew M. Demchuk, MD, University of Calgary, Calgary, Alberta, Canada. “The heterogeneity and time-dependent nature of stroke have greatly limited our success in advancing stroke treatments.” Prof. Demchuk suggested better patient selection using advanced neurovascular imaging techniques, using a combination of systemic thrombolysis treatments, and earlier, more frequent, and more complete recanalization as solutions.
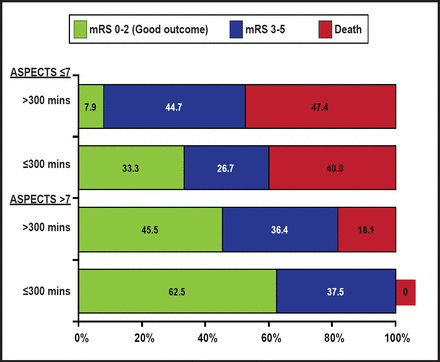
Patients with a favorable baseline CT scan appearance are the most likely to benefit from IV intra-arterial therapy [Hill MD et al. Am J Neuroradiol 2006]. There is also evidence that good clinical outcome following angiographically successful reperfusion is significantly time-dependent. At later times, angiographic reperfusion may be associated with a poor risk-benefit ratio in unselected patients [Khatri P et al. Neurology 2009]. Overall, patients benefit the most with early recanalization and a favorable baseline CT scan (ASPECTS score >7; Figure 1) [Goyal M et al. Stroke 2011]. CT-angiogram has additional information regarding extent of thrombus burden and collateral flow, which are key determinants of whether an artery will successfully recanalize and whether a brain that is at risk will go on to infarction. A simple noncontrast CT and CTA approach requires the least amount of time yet provides advanced imaging information for the clinician, using up far fewer of the 1.9 million neurons that are lost with each minute of a stroke.
NCCT ASPECTS and Time to Recanalization.
Reproduced with permission from A. Demchuk, MD.
“Time is brain,” Prof. Demchuk reminded the audience.
- © 2011 MD Conference Express