Summary
Results from the Clazosentan in Reducing Vasospam-Related Morbidity and Mortality in Adult Patients with Aneurysmal Subarachnoid Hemorrhage Treated By Surgical Clipping [CONSCIOUS-2; NCT00558311] study indicate that the use of the endothelin receptor antagonist clazosentan does not significantly improve vasospasm-related morbidity or all-cause mortality after aneurysmal subarachnoid hemorrhage.
- Ischemia
- Cerebrovascular Disease
Results from the Clazosentan in Reducing Vasospam-Related Morbidity and Mortality in Adult Patients with Aneurysmal Subarachnoid Hemorrhage Treated By Surgical Clipping (CONSCIOUS-2; NCT00558311) study, presented by Robert Loch Macdonald, MD, PhD, St. Michael's Hospital, University of Toronto, Toronto, Ontario, Canada, indicate that the use of the endothelin receptor antagonist clazosentan does not significantly improve vasospasm-related morbidity or all-cause mortality after aneurysmal subarachnoid hemorrhage (aSAH).
Cerebral vasospasm is a frequent complication of aSAH that leads to significant morbidity and mortality. In a previous study [R. Loch Macdonald et al. Stroke 2008], clazosentan significantly and dose-dependently reduced angiographic vasospasm after aSAH, with a trend toward a reduction in morbidity and mortality. CONSCIOUS-2 was designed to assess whether clazosentan reduces cerebral vasospasm-related morbidity and all-cause mortality within 6 weeks post-aSAH.
This was a randomized, double-blind, placebo-controlled study of men and women aged 18 to 75 years with a ruptured saccular aneurysm, confirmed by angiography and successfully secured by surgical clipping, for which the time of rupture was known or estimated with a reasonable degree of certainty. Subjects were required to have a World Federation of Neurological Surgeons (WFNS) grade I-IV prior to the clipping procedure that did not worsen to grade V postprocedure (based on regular Glasgow Coma Scale) and a diffuse subarachnoid clot on the baseline computed tomography (CT) scan.
Eligible subjects were randomly assigned 2:1 to receive intravenous clazosentan (5 mg/hour; n=764) or placebo (n=383), starting within 56 hours of clipping and continuing for up to 2 weeks. The primary composite endpoint was vasospasm-related morbidity and all-cause mortality within 6 weeks of aSAH (ie, death, new cerebral infarcts due to vasospasm, delayed ischemic neurological deficit [DIND] due to vasospasm, and/or the use of rescue therapy). The main secondary endpoints were functional clinical outcome, as measured by extended Glasgow Outcome Scale (GOSE), dichotomized into good (score >4) and poor (score ≤4), at Week 12; total volume of new cerebral infarcts of all etiologies at Week 6; and the individual components of the primary endpoint. Safety was assessed by adverse events.
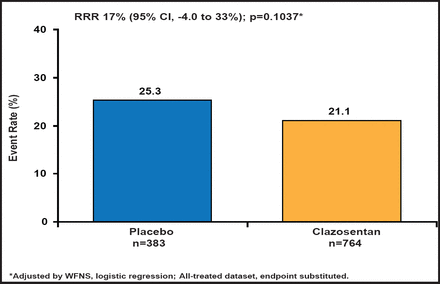
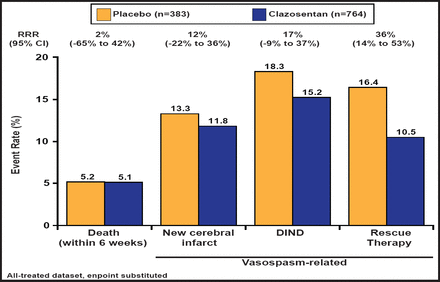
Subjects had a mean age of 52 years, and about 70% were women. Most (∼77%) were WFNS class I–II. Approximately 50% of the subjects had diffuse thick hemorrhages. There was a nonsignificant 17% reduction in the primary endpoint in the treated group (Figure 1). There was a trend toward poor outcomes (GOSE score ≤4) in the clazosentan-treated group (29.3%) versus placebo-treated patients (24.8%; RRR, −18%; 95% CI, −45% to 4%; p=0.1048, logistic regression adjusted for WFNS). When the individual endpoint components were analyzed separately, clazosentan had no effect on mortality; however, drug treatment did reduce the formation of new vasospasm-related cerebral infarcts, the occurrence of vasospasm-related DIND, and the use of rescue therapy, although none of these effects was significant (Figure 2). In subgroup analyses, clazosentan reduced mortality/vasospasm-related morbidity in patients with poor WFNS grade (≥III) (RRR, 33%; 95% CI, 8% to 51%) and diffuse thick SAH (diffuse thick clot on baseline [CT] scan; RRR, 25%; 95% CI, 5% to 41%); however, no effect was observed on GOSE. Rescue therapy, a primary endpoint component, was used in 11% of clazosentan-treated and 16% of placebo-treated patients.
Vasospasm-Related Morbidity/All-Cause Mortality Within 6 Weeks
Reproduced with permission from RL Macdonald, MD, PhD.
Individual Components of the Composite Endpoint.
Reproduced with permission from RL Macdonald, MD, PhD.
Treatment-emergent lung complications, anemia, and hypotension occurred in 34%, 22%, and 12% of clazosentan-treated patients versus 18%, 15%, and 4% of placebo-treated patients, respectively. Mortality was similar between the two groups (6%).
- © 2011 MD Conference Express