Summary
Until the recent Rythmol Atrial Fibrillation [RAFT] Trial, it was unclear whether cardiac resynchronization therapy was beneficial in patients with mild to moderate congestive heart failure (HF). The cumulative evidence to date indicates that the addition of CRT to optimal medical or defibrillator therapy significantly reduces mortality among patients with HF [Wells G et al. CMAJ 2011].
- Heart Failure
- Interventional Techniques & Devices
Until the recent Rythmol Atrial Fibrillation (RAFT) Trial, it was unclear whether cardiac resynchronization therapy (CRT) was beneficial in patients with mild to moderate congestive heart failure (HF). The cumulative evidence to date indicates that the addition of CRT to optimal medical or defibrillator therapy significantly reduces mortality among patients with HF [Wells G et al. CMAJ 2011]. John Cleland, MD, University of Hull, Kingston-upon-Hull, UK, discussed the role and guidelines for CRT therapy in HF patients.
Current guidelines state that patients who receive these devices should have a left ventricular ejection fraction (LVEF) ≤35%, a QRS duration ≥0.12 seconds, and sinus rhythm; be NYHA functional Class III; and already be receiving optimal medical therapy. According to Prof. Cleland, patients with HF who require an implantable defibrillator should have CRT routinely [Cleland JG et al. Heart 2008]. CRT is useful because it can sense and pace the right atrium, shorten the atrioventricular interval, pace the right ventricle (RV) and the left ventricle (LV), alter the timing of RV and LV free-wall contraction and relaxation, raise systolic blood pressure, and improve hemodynamics.
Despite major advances in cardiac treatment, HF remains a progressive and fatal disease. Mark S. Slaughter, MD, University of Louisville, Kentucky, USA, described the implantable ventricular assist devices that are available as a bridge to heart transplantation for patients with advanced HF.
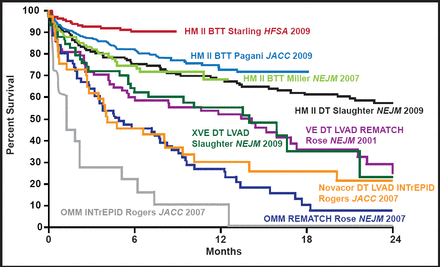
Survival rates >90% out to 1 year and improvements in functional status, quality of life, and 6-minute walk test have been achieved with continuous flow left ventricular assist devices (LVADs) in patients who are awaiting transplantation (Figure 1). These improvements are equal to, if not better than, those that are achieved with heart transplantation at 1 year for patients in the most critical categories. Adverse events have also been significantly reduced. With increased wait times for transplants, it is unlikely that patients would survive to transplant without the use of these devices. Based on the significant advances in the last several years, Dr. Slaughter believes continuous flow LVADs should be used earlier in patients with advanced HF for both bridge to transplantation or for permanent use (ie, destination therapy).
Improving Survival in LVAD Trials.
Reproduced with permission from M. Slaughter, MD.
Andreas M. Zeiher, MD, University of Frankfurt, Frankfurt, Germany, believes that there is now evidence that the heart is a regenerating organ. The capacity to generate cardiomyocytes in healthy and injured hearts suggests that it may be rational to work toward the development of therapeutic strategies that are aimed at stimulating this process in cardiac pathologies [Bergmann O et al. Science 2009]. Beyond conventional therapy, evidence suggests that adult bone marrow-derived cell transplantation is associated with modest improvements in physiological and anatomical parameters in patients with both acute myocardial infarction (AMI) and chronic ischemic heart disease [Abdel-Latif A et al. Arch Intern Med 2007]. The mechanism of action includes effects on vasculogenesis, paracrine effects, and cardiomyogenesis. Although the effects are modest, enhanced strategies, such as shockwave-facilitated cell therapy, may improve efficacy, at least for AMI. There is a lack of data to recommend this treatment for chronic postinfarction HF.
“The management of volume overload in advanced heart failure is one of the most difficult areas for physicians,” said Barry M. Massie, MD, University of California, San Francisco, California, USA. Although loop diuretics are at the core of pharmacological management of volume overload in chronic HF, current treatments have issues. Furosemide has a highly variable oral bioavailability (20% to 60%) and is short-acting. Bumetanide is relatively short-acting but is safe in patients with a sulfa allergy. Torsemide has a high oral bioavailability (60% to 80%) and a long duration of action.
Recent evidence with furosemide therapy in patients with acute decompensated heart failure (ADHF) demonstrated no significant difference in patients' global assessment of symptoms with a high-dose strategy of loop diuretic within the first 3 days of ADHF compared to a more conservative low-dose strategy. The Diuretic Optimization Strategies Evaluation (DOSE) trial [Felker GM et al. N Engl J Med 2011] compared various diuretic strategies for patients with ADHF. Patients were randomly assigned, in a 1:1:1:1 ratio, to either a low-dose strategy (total intravenous furosemide dose equal to their total daily oral loop diuretic dose in furosemide equivalents) or a high-dose strategy (total daily intravenous furosemide dose 2.5 times their total daily oral loop diuretic dose in furosemide equivalents) and to administration of furosemide either by intravenous bolus every 12 hours or by continuous intravenous infusion. In comparison of bolus with continuous infusion, there were no significant differences in the co-primary endpoints of patients' global assessment of symptoms (mean AUC, 4236±1440 and 4373±1404, respectively; p=0.47) or in the mean change in the creatinine level (0.05±0.3 mg/dL [4.4±26.5 μmol/L] and 0.07±0.3 mg/dL [6.2±26.5 μmol/L], respectively; p=0.45). In the comparison of the high-dose strategy with the low-dose strategy, there was a nonsignificant trend toward greater improvement in patients' global assessment of symptoms in the high-dose group (mean AUC, 4430±1401 vs. 4171±1436; p=0.06). There was no significant difference between these groups in the mean change in the creatinine level (0.08±0.3 mg/dL [7.1±26.5 μmol/L] with the high-dose strategy and 0.04±0.3 mg/dL [3.5±26.5 μmol/L] with the low-dose strategy, p=0.21). Although there was no clear benefit from a high-dose strategy on the prespecified primary endpoints, a high-dose strategy was associated with greater diuresis and more favorable outcomes in some secondary measures, but also with transient worsening of renal function.
Intravenous loop diuretics are the mainstay therapy for patients with ADHF. However, because of complications, such as diuretic resistance and worsening renal function, other treatments, especially adjunctive use of aldosterone blockers (spironolactone, eplerenone), are often useful, both in the short- and long-term. The combination of loop and thiazide diuretics can be very effective, but the magnitude of diuresis is unpredictable and may be excessive. This combination should be initiated in inpatients and only maintained postdischarge after the effect in individual patients has been characterized. Dr. Massie recommended that it is best used every other day or less as needed because of the potential for excessive diuresis and electrolyte abnormalities. Addition of an adenosine antagonist (aminolphylline is one such drug) has been used in this setting and has the potential to overcome diuretic resistance, but recent trials have not shown the expected benefit.
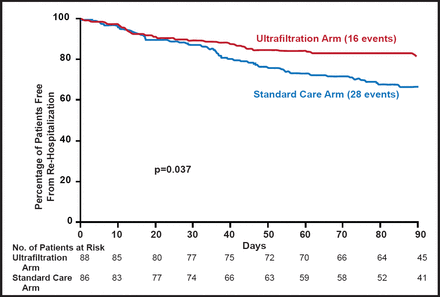
Another approach is ultrafiltration, which can remove fluid from the blood at the same rate that fluid can be naturally recruited from the tissue. The transient removal of blood elicits a compensatory mechanism, called plasma or intravascular refill (PR). In decompensated HF, ultrafiltration safely produces greater weight and fluid loss than intravenous diuretics, reduces 90-day resource utilization for HF, and is an effective alternative therapy (Ultrafiltration vs IV Diuretics for Patients Hospitalized for Acute Decompensated CHF [UNLOAD; NCT00124137]) (Figure 2) [Costanzo MR et al. J Am Col Cardiol 2007]. The Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS; NCT00608491) study of ultrafiltration versus diuretics in patients with ADHF is in the process of confirming these findings.
Freedom from Rehospitalization for HF.
Reprinted from J Am Col Cardiol. Constanza MR et al. Feb 13, 2007;49(6):675–683. With permission from Elsevier.
- © 2011 MD Conference Express