Summary
Investigators from the Efficacy Study of Drug-Eluting and Bare Metal Stents in Bypass Graft Lesions [ISAR-CABG; NCT00611910] report that treatment of saphenous vein graft lesions with drug-eluting stents may decrease the long-term need for repeat target lesion revascularization by approximately 50% compared with bare metal stents, making DES the preferred way to reduce restenosis in bypass grafts.
- Interventional Techniques & Devices Clinical Trials
Investigators from the Efficacy Study of Drug-Eluting and Bare Metal Stents in Bypass Graft Lesions (ISAR-CABG; NCT00611910) report that treatment of saphenous vein graft (SVG) lesions with drug-eluting stents (DES) may decrease the long-term need for repeat target lesion revascularization (TLR) by approximately 50% compared with bare metal stents (BMS), making DES the preferred way to reduce restenosis in bypass grafts.
Only two published randomized controlled trials have compared DES with BMS for SVG lesions: the DELAYED RRISC (Reduction of Restenosis in Saphenous Vein Grafts With Sirolimus-Eluting Stent) trial [Vermeersch P et al. J Am Coll Cardiol 2007] and the SOS (Stenting of Saphenous Vein Grafts) trial, which compared paclitaxel-eluting stents (PES) with BMS [Brilakis ES. J Am Coll Cardiol 2009]. The results of these trials were mixed, and neither was powered to examine clinical endpoints (DELAYED RRISC trial n=75; SOS trial n=80).
In the ISAR-CABG trial, patients with de novo SVG lesions were randomized to receive either DES (sirolimus, paclitaxel, or biodegradable polymer-based sirolimus) or BMS. Participants had ischemic symptoms or evidence of myocardial ischemia in the presence of ≥50% de novo stenosis that was located in SVGs. Exclusion criteria included cardiogenic shock, target lesions in arterial grafts, malignancies with <1 year of life expectancy, and allergies to study medication. The primary endpoint of the prospective ISAR-CABG trial was a composite of death, myocardial infarction (MI), or ischemia-related TLR at 1-year postindex percutaneous coronary intervention (PCI). Secondary endpoints included all-cause mortality, MI, ischemia-related TLR, and incidence of definite or probable stent thrombosis. Patients were followed for 12 months, with a repeat angiogram that was encouraged at 6 to 8 months. After PCI, patients were treated with clopidogrel 150 mg per day until discharge, followed by 75 mg clopidogrel daily for at least 6 months and aspirin 200 mg daily indefinitely.
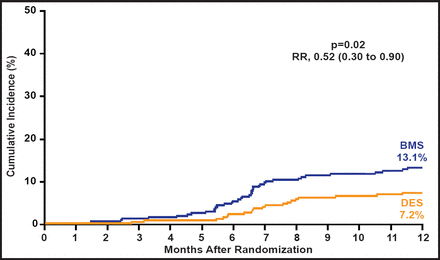
A total of 610 patients were randomized (DES=303, BMS=307) and baseline characteristics were balanced between groups. The primary composite endpoint of death, MI, or TLR at 12 months was significantly reduced with DES compared with BMS (15.4% vs 22.1%; RR 0.65; 95% CI, 0.45 to 0.96; p=0.03), driven primarily by a reduction in TLR (7.2% DES vs 13.1% BMS; RR, 0.52; 95% CI, 0.30 to 0.90; p=0.02; Figure 1). There was no significant difference in the rates of MI (p=0.27) or mortality (p=0.82). There was no difference in the rate of definite or probable stent thrombosis between groups (0.7% both groups; p=0.99).
Target Lesion Revascularization.
Reproduced with permission from J. Mehilli, MD.
The ISAR-CABG outcomes show that DES are associated with lower rates of MACE compared with BMS for SVG lesions. These results are driven largely by a reduced need for revascularization, and there were no significant differences in death or stent thrombosis between groups. These findings are supportive of those that are found in the long-term follow-up of the SOS trial, which showed that the use of paclitaxel-eluting stents was associated with significantly better clinical outcomes than BMS in SVG lesions (NCT00247208) [Emmanouil S et al. J Am Coll Cardiol Cardiovasc Interv 2011].
- © 2011 MD Conference Express