Summary
A wide variety of drugs can “tip the balance of glucose homeostasis,” leading to dysglycemia. But, the risks and benefits of these drugs should be carefully considered, as their benefits may outweigh an increased risk of diabetes. This article discusses thiazide-induced dysglycemia, the association of statins with new-onset diabetes, the relationship between diabetes and mental illness, and strategies to prevent drug-induced dysglycemia.
- Lipid Disorders
- Hyperglycemia/Hypoglycemia
- Psychopharmacology
A wide variety of drugs can “tip the balance of glucose homeostasis,” leading to dysglycemia, said Charles D. Ponte, PharmD, DPNAP, FAPhA, FASHP, FCCP, West Virginia University School of Pharmacy, Morgantown, West Virginia, USA. But, the risks and benefits of these drugs should be carefully considered, as their benefits may outweigh an increased risk of diabetes.
Dr. Ponte listed several drugs that cause either hyperglycemia or hypoglycemia, noting that the list was in no way complete (Table 1). Drugs alone may alter blood glucose levels, but in many cases, the drugs can interact with a variety of patient factors that could, for example, result in altered drug metabolism or some other consequence, leading to hyperglycemia or hypoglycemia. Among the risk factors are a history of metabolic syndrome or prediabetes, older age, polypharmacy, and reduced carbohydrate intake. The consequences of drug-induced dysglycemia may be no different than the morbidity and mortality that are caused by hyperglycemia and hypoglycemia from any cause [Dang DK, Pucino F, Ponte CD, Calis KA. Glucose and insulin dysregulation. In: Tisdale JE, Miller DA. Drug-Induced Diseases. 2nded. Bethesda, MD: American Society of Health-System Pharmacists, Inc; 2010].
Examples of Drugs that Cause Dysglycemia.
Before determining that dysglycemia is drug-induced, clinicians should consider what other conditions may have a role in causing abnormal glucose levels. The differential diagnosis includes diabetes, pancreatitis, cirrhosis, Addison disease, alcoholism, glycogen storage disease, and others.
Some drugs of particular concern in the diabetes setting are two commonly used drugs—thiazide diuretics and statins—and antipsychotic agents, all of which have been associated with an increased risk of diabetes.
Thiazide Diuretics
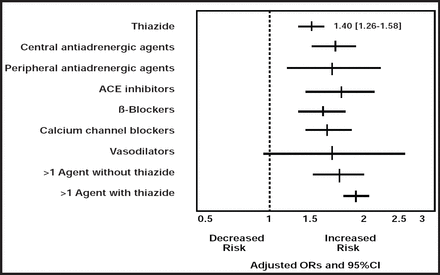
Thiazide-induced dysglycemia has generated a long-standing debate, said Michael Ernst, PharmD, BCPS, FCCP, University of Iowa, Iowa City, Iowa, USA. Early researchers warned against the use of the drugs, but a later study showed that while thiazide was associated with a 40% increased risk of hyperglycemia, almost all antihypertensive drugs were associated with an increased risk (Figure 1) [Gurwitz JH et al. Ann Intern Med 1993]. A combination of antihypertensive agents, one of which was a thiazide, was associated with the greatest risk.
Risk of Hyperglycemia with the Use of Antihypertensives.
Reproduced with permission from the American College of Physicians, from Antihypertensive Drug Therapy and the Initiation of Treatment for Diabetes Mellitus, Gurwitz JH et al Annals Internal Medicine; vol. 118, no. 4, 273–278, 1993; permission conveyed through Copyright Clearance Center, Inc.
In some subsequent hypertension trials, there was a 20% to 50% increase in new-onset diabetes that was associated with thiazide, although the development of diabetes was not a defined endpoint. One exception was the Atherosclerosis Risk in Communities (ARIC) study, in which thiazide was not associated with an increased risk of diabetes (HR=0.95), but β-blockers were (HR=1.26) [Gress TW et al. N Engl J Med 2000]. Since diuretics were often used with β-blockers in the earlier studies, exactly how much of the risk of diabetes to attribute specifically to thiazides is uncertain. Many questions remain about how the various antihypertensive agents work, and a better understanding of the side effects of these agents could help clarify their association with hyperglycemia.
Small, short-term studies have shown that thiazide appears to worsen glycemic control in a dose-dependent manner by reducing insulin secretion and peripheral insulin sensitivity. In addition, long-term observational studies in patients who are treated with antihypertensives have shown an inverse relationship between potassium and glucose levels. Taken together, the data suggest that thiazide impairs the potassium-mediated release of insulin. However, potassium probably does not account for all of the risk, and there has not been adequate study to determine if managing potassium levels will prevent dysglycemia.
No increased harm has been found with diabetes that develops during treatment of hypertension with thiazide. In addition, the time to the development of diabetes has been shorter among subjects with uncontrolled hypertension than those with controlled hypertension [Izzo R et al. Diabetes Care 2009]. These findings indicate that clinicians should control blood pressure in their patients with hypertension while aggressively controlling other cardiovascular risk factors, particularly in patients who are at risk for the development of diabetes.
Dr. Ernst emphasized, “Diuretics remain valuable agents for blood pressure control and preventing cardiovascular disease-related events. Fear of their dysglycemic effects should not be reason to avoid them.”
Statins
Early data on the association of statins with new-onset diabetes is conflicted. In a study that compared pravastatin with placebo for primary prevention (WOSCOPS), the statin was associated with a 30% decrease in the incidence of new-onset diabetes (p=0.042), but the number of people developing diabetes were small [Freeman DJ et al. Circulation 2001]. Three of four subsequent major statin trials (ASCOT-LLA, CORONA, HPS) that included data on new-onset diabetes suggested a possible increased risk (about 14% to 15%), while the fourth showed no difference (LIPID). The “game changer” was the JUPITER trial, said David Preiss, FRCPath, MRCP, BHF Glasgow Cardiovascular Research Centre, University of Glasgow, Scotland, United Kingdom. New-onset diabetes was a specified secondary endpoint in this large trial (n=17,802) of rosuvastatin versus placebo for primary prevention, and the statin was associated with a 25% increase in diabetes (270 cases vs 216 cases in the control group; p=0.01) [Ridker PM et al. N Engl J Med 2008].
The data from these studies led Dr. Preiss and colleagues to conduct a collaborative meta-analysis on 13 randomized placebo- and standard care-controlled trials, in which they combined published and unpublished data. The results demonstrated a 9% increased relative risk of diabetes that was associated with statins, with a modest absolute risk of only 1 extra case of diabetes per 1000 patient-years [Sattar N et al. Lancet 2010]. The important point, said Dr. Preiss, was the clear benefit of the statins in reducing cardiovascular disease-related events (Table 2).
Risk-Benefit Considerations with Statins.
Trial data suggested the possibility that intensive statin regimens may be associated with a somewhat greater risk. For example, in the JUPITER trial, the risk of incident diabetes was 26% higher than in the placebo group, and in the SPARCL trial, the risk was increased 44% with atorvastatin 80 mg versus placebo [Waters DD et al. J Am Coll Cardiol 2011]. In JUPITER, the absolute risk was 7 new cases of diabetes per 10 patients who were protected from major cardiovascular (CV) events (CV death, stroke, and myocardial infarction) with rosuvastatin. In SPARCL, there was an approximate absolute risk of 9 new cases of diabetes per 10 patients who were protected from a CV event with atorvastatin [SPARCL Investigators. N Engl J Med 2006]. A recent pooled analysis of five statin trials, involving 32,752 patients without diabetes at baseline but with a high risk for CV events, showed that high-dose statins were associated with a 12% increase in the risk of diabetes compared with low-dose statins [Preiss D et al. JAMA 2011], in keeping with a dose-dependent effect. At the same time, high-dose statins led to a 16% reduction in major CV events compared with low-dose statins. Overall benefit was still clear in this high-risk group, although there was one additional case of diabetes for every three patients who were protected from a major CV event.
These findings indicate that clinicians should continue to follow recommended statin prescribing, and there are no changes that are needed for the treatment of patients with established diabetes. It appears sensible to screen patients who are taking statins for diabetes, especially for those with intensive statin regimens. Perhaps most important, Dr. Preiss added, is to inform patients of the link between new-onset diabetes and statins, so that patients can weigh the benefits and risks and participate in decision-making.
Antipsychotic Drugs
Severe mental illness is associated with a 2- to 3-fold increase in the prevalence of diabetes; this association was recognized over a century ago in the preantipsychotic era. However, following the introduction of antipsychotic medication in the 1950s, a number of reports that link antipsychotics and diabetes mellitus and/or impaired glucose tolerance have emerged. This association came to be generally accepted by the late 1960s, and the term “phenothiazine diabetes” was coined [Thonnard-Neumann E. Am J Psychiatr 1968].
There are a number of potential mechanisms for dysglycemia with antipsychotic drugs, including weight gain, insulin resistance, and β-cell failure, said Richard I.G. Holt, PhD, FRCP, University of Southampton School of Medicine, Southampton, United Kingdom. Such side effects have been reported to be more common with second-generation antipsychotics. For example, olanzapine and clozapine have been associated with greater weight gain compared with placebo and first-generation antipsychotics as well as other second-generation antipsychotics. In a meta-analysis of a series of head-to-head randomized controlled trials, olanzapine has also been associated with greater increases in serum glucose, weight gain, and dyslipidemia than other second-generation antipsychotics, with the exception of clozapine [Rummel-Kluge C. Schizophr Res 2010].
The increased risk of dysglycemia and/or diabetes calls for clinicians to screen for diabetes and to educate their patients who take antipsychotics about the need for lifestyle modifications. Screening guidelines have been published in both the United States and Europe; these guidelines recommend determining a baseline fasting or random glucose level (or HbA1C level) before antipsychotic treatment begins, with follow-up levels determined at 3 to 4 months and then annually [De Hert M et al. Eur Psychiatry 2009; ADA. Diabetes Care 2004].
The risk of diabetes must be balanced with the benefits of treatment with antipsychotic drugs, added Prof. Holt. Before deciding to stop an antipsychotic, the potential role of the antipsychotic, the duration of treatment, and other risk factors must be weighed against the risk of relapse and the benefits of treatment. If diabetes does develop, the management of schizophrenia or bipolar illness and diabetes requires a multidisciplinary approach.
Preventing Drug-Induced Dysglycemia
Dr. Ponte described several strategies to prevent drug-induced dysglycemia (Table 3). He emphasized the need for clinicians to monitor a patient when interacting drugs cannot be avoided, adding that if an interaction results in a morbid event, clinicians should reduce the dose or discontinue the offending drug(s) and manage the hyperglycemia or hypoglycemia appropriately.
Strategies to Prevent Drug-Induced Dysglycemia.
- © 2011 MD Conference Express