Summary
Intravenous (IV) insulin is currently the standard of care but requires frequent monitoring and can cause excess hypoglycemia. This article presents results from a pilot study to determine the feasibility, efficacy, and safety of IV exenatide in hyperglycemic cardiac intensive care unit patients [Intravenous Exenatide in Coronary Intensive Care Unit Patients; NCT00736229].
- Insulin
- Diabetes Mellitus
- Diabetes & Endocrinology Clinical Trials
In critically ill patients, persistent hyperglycemia is associated with increased mortality and complications [Kosiborod M et al. Circulation 2005], but low blood glucose is equally dangerous [Marik P. World J Gastrointest Surg 2009]. Intravenous (IV) insulin is currently the standard of care but requires frequent monitoring and can cause excess hypoglycemia. Steven P. Marso, St. Luke's Mid America Heart & Vascular Institute, Kansas City, Missouri, USA, presented results from a pilot study to determine the feasibility, efficacy, and safety of IV exenatide in hyperglycemic cardiac intensive care unit (CICU) patients [Intravenous Exenatide in Coronary Intensive Care Unit Patients; NCT00736229].
Dr. Marso and his colleagues performed a prospective, single-center, open-label, nonrandomized study that compared IV exenatide to insulin controls. The primary outcome measure was average glucose value during a coronary ICU stay of 24 to 48 hours. Secondary outcome measures included number of hypoglycemic episodes in the ICU, number of subjects with >1 ICU hypoglycemic episode or serious adverse event (death, life-threatening event, prolonged hospital stay, disability or incapacity, non-life-threatening event) within 30 days of the discontinuation of the study drug.
Eligibility criteria included age >18 years; admission to the CICU; admission blood glucose (BG) of 140 to 400 mg/dL; primary cardiovascular diagnosis by the attending physician; being under the primary care of the cardiology service; ventilator independence; and the ability to provide informed consent. Exclusion criteria included creatinine clearance <30 mL/min, type 1 diabetes, pregnancy, gastroparesis, insulin treatment (except monotherapy for long-acting basal insulin), admittance to the CICU to measure hemodynamics prior to transplant, or posttransplant procedure.
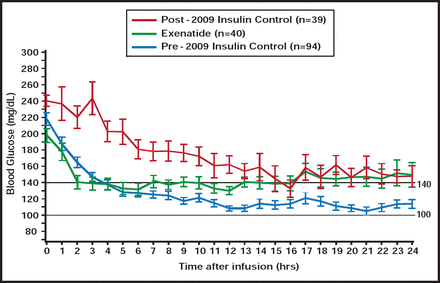
Exenatide was infused at a fixed dose of 0.05 mcg/min (30-min bolus), then 0.025 mcg/min continuously for 24 to 48 hrs. The drug was benchmarked to 2 insulin control groups: 1) intensive (INT; target BG 90 to 119 mg/dL) and 2) modified (MOD; target BG 100 to 140 mg/dL). Exenatide was infused in 40 patients (age 65 years, 83% male, 63% acute coronary syndromes, 75% type 2 diabetes). Admission BG was 199.3±52.7 mg/dL in exenatide patients and 240.3±44.0 mg/dL in the MOD group (p=0.02). Time to target BG was lower in the exenatide than MOD group (3.9±4.3 vs 9.3±7.4 hours; p<0.001; Figure 1). Drug-related nausea occurred in 8 (20%) exenatide patients; 5 (13%) discontinued use early. Exenatide was associated with a lower BG than MOD.
Mean Glucose: 3 Groups.
Reproduced with permission from S. Marso, MD.
Of 668 and 745 glucose observations in the exenatide and MOD groups, respectively, there were numerically fewer overall hypoglycemic episodes in the exenatide group (0.9% vs 1.2%; p=0.57), including severe events (0% vs 0.3%; p=0.18). BG in exenatide-treated patients was more frequently within the target range (100 to 140 mg/dL; 37% vs 29%; p<0.001) and within 71 to 140 mg/dL (48% vs 39%; p<0.001) compared with MOD. No serious adverse events were observed in the exenatide group.
The study objective was to determine the feasibility, efficacy, and safety of glucose-lowering with IV exenatide monotherapy in hyperglycemic patients who were admitted to the CICU. The findings suggest that fixed-dose IV exenatide is feasible in hyperglycemic ICU patients, achieves similar efficacy compared with IV insulin, and does not cause severe hypoglycemia.
- © 2011 MD Conference Express