Summary
There are three major accepted indications for transcatheter aortic valve implantation (TAVI), as well as a number of minor conditions in which TAVI may be reasonable. The first is an inoperable patient with severe aortic stenosis (mean gradient >40 mm Hg or jet velocity >4 m/sec) and surgical risk >50%. The benefit of TAVI in these patients was shown in the PARTNER trial [NCT00530894; Leon MB et al. N Engl J Med 2010], which reported a cardiovascular mortality rate after 1 year of 20.5% for TAVI patients versus 44.6% for patients who received standard therapy (p<0.001).
- Valvular Disease
- Interventional Techniques & Devices
There are three major accepted indications for transcatheter aortic valve implantation (TAVI), as well as a number of minor conditions in which TAVI may be reasonable, noted Gerhard Schuler, MD, University of Leipzig, Leipzig, Germany. The first is an inoperable patient with severe aortic stenosis (mean gradient >40 mm Hg or jet velocity >4 m/sec) and surgical risk >50%. The benefit of TAVI in these patients was shown in the PARTNER trial [NCT00530894; Leon MB et al. N Engl J Med 2010], which reported a cardiovascular mortality rate after 1 year of 20.5% for TAVI patients versus 44.6% for patients who received standard therapy (p<0.001).
The second condition includes patients with severe aortic stenosis (AVA <0.8 cm2, AVG >40 mm Hg, peak jet velocity ≥4.0 m/sec), NYHA class ≥II, and surgical risk >15%. Support for this indication again comes from the PARTNER trial, which showed that transcatheter replacement was not inferior to surgical replacement. All-cause mortality at 1 year was 26.8% for standard therapy and 24.2% for TAVI (HR, 0.93; 95% CI, 0.71 to 1.22; p=0.62 for non-inferioriy) [Smith CR et al. N Engl J Med 2011].
The third possible group that might benefit from TAVI includes patients with degenerated bioprosthetic aortic valve and severe aortic stenosis (AVA <0.8 cm2, AVG >40 mm Hg, peak jet velocity >4.0 m/sec). However, there are no current prospective data evaluating the risks and benefits of TAVI in this group of patients.
Other clinical scenarios in which TAVI may be reasonable include patients with porcelain aorta, frail patients, those with pulmonary fibrosis or patent coronary bypass grafts, post chest radiation, and patients with liver cirrhosis. Lastly, patients with low-flow/low-gradient aortic stenosis might benefit from TAVI. This list is not exhaustive and is likely to grow in the future.
The current choices for treating aortic stenosis in patients who are considered to be non-surgical candidates are medical treatment, balloon aortic valvuloplasty (BAV), and TAVI. Josep Rodés-Cabau, MD, Quebec Heart & Lung Institute, Quebec City, Quebec, Canada, discussed how to choose the right treatment approach for these patients. In the pre-TAVI era, BAV was used as a palliative treatment in those patients who were not candidates for standard aortic valve replacement (SAVR). The procedure was associated with a modest improvement in valve hemodynamics and a relatively low complication rate (stroke ∼1.5%, major vascular complications ∼4%, mortality ∼1.5%). TAVI has emerged as the treatment of choice for non-surgical candidates to SAVR. The PARTNER trial randomized these patients to TAVI versus medical treatment but up to 83% of the patients in the medical group received BAV as a palliative treatment. Despite a higher rate of periprocedural complications, such as stroke and major vascular complications, TAVI was associated with a significant reduction in global mortality, cardiovascular mortality, and re-hospitalization at the 1-year follow-up. TAVI also showed superior results compared with BAV in valve hemodynamics, functional status, and quality of life.
BAV can be used nowadays as a bridge to TAVI in patients who are at very high-risk of periprocedural complications and those with uncertain response to TAVI (very low left ventricular ejection fraction, severe pulmonary hypertension, mitral regurgitation or chronic obstructive pulmonary disease, and extremely frail patients).
Despite the good results that are associated with TAVI, not all patients who are considered non-operable are good candidates for TAVI. In fact, the mortality rate at the 1-year follow-up for TAVI is about 15% to 30% and about 20% of the patients do not improve their functional status or quality of life. Future research efforts should be done to better identify the factors that are associated with a poor response to TAVI, and this should contribute to the improvement of patient selection and the results that are associated with TAVI.
Although TAVI is now recommended for high risk patients, questions remain whether it is suitable for patients with low surgical risk. Surgical aortic valve replacement in this group is associated with low risk and excellent outcomes over long-term follow up. According to Nicolo Piazza, MD, German Heart Center, Munich, Germany, the answer is no, “but if we do perform TAVI in these patients, it should be under randomized controlled settings.”
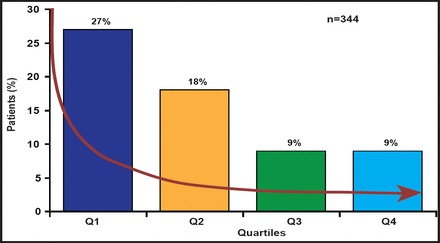
The BERMUDA Triangle Study that is being conducted at Bern University Hospital, German Heart Centre, Munich, Germany, and Erasmus University Medical Center, Rotterdam, the Netherlands, is a matched cohort study consisting of 392 patients (stratified according to STS [Society of Thoracic Surgeons] score) each receiving TAVI or SAVR. The majority of patients (n=510) had an STS score of between 3% and 8 %. However, as TAVI replaces mechanical and biological SAVR as the procedure of choice, it is lowering the age, logistic EuroSCORE, and STS score of patients who are being considered for treatment. Rates of 30-day and 6-month mortality have dropped 4-fold and 2-fold, respectively, during the 3-year period of the study. These changes have been attributed to patient-, procedural- or operator-, and device-related factors. Despite decreasing vascular complications as operators become more technically proficient with percutaneous closure devices (Figure 1), Prof. Piazza is hesitant to recommend TAVI for low-risk patients. His concerns include stroke, paravalvular regurgitation, vascular injury, durability, conduction abnormalities, and lack of formal health technology assessment. Ultimately, a well-designed randomized trial will be necessary to answer these questions.
Vascular Complications.
Reproduced with permission from N. Piazza, MD.
Redo SAVR surgery is the standard treatment for failed surgical bioprostheses, but may lead to high mortality and morbidity in the presence of comorbidities. Dominique Himbert, MD, Bichat Hospital, Paris, France, discussed TAVI as an option to SAVR for patients at who are high risk or with contraindications to surgery (eg, high-risk elderly patients with degenerated bioprostheses).
In high-risk patients, the less invasive TAVI approach may be safer than redo SAVR. The avoidance of technical difficulties (adhesions, patent grafts) and shortening of hospital stay and rehabilitation are other reasons for its use. The key to procedural success lies in paying attention to the same general principles as those that are used for TAVI in native valves including no predilatation, correct orientation of the valve on the balloon, the positioning of the transcatheter heart valve (depending on which device is used), rapid ventricular pacing, and balloon inflation (use a slow two-step inflation process to correct and adjust the position of the valve within the surgical bioprothesis).
There are a number of favorable case reports that involve the use of TAVI within a surgically implanted failed bioprosthesis, mainly using the Edwards SAPIEN device via transapical access. The data are preliminary and require further confirmation by larger series and longer follow-ups. There are a number of issues to resolve such as variability and discrepancies in the sizing of surgical bioprostheses, how to deal with coronary obstruction, the prostheses that are dedicated for valve-in-valve (smaller sizes, outer basal cloth, and supravalvular prostheses are needed) as well as durability before there is a wide acceptance of this procedure.
The positive outcomes that are associated with TAVI in selected patients who are treated at centers with the appropriate capacity to perform these procedures are encouraging. While the use of TAVI will likely increase as experience with this procedure grows, it will be challenging for clinicians to guide patients who fall outside of the populations that have been studied in randomized trials to the best procedural option. Improving technologies and procedural techniques will likely add further complexity. Additional randomized comparisons are necessary.
The editors would like to thank the many members of the ESC Congress 201 presenting faculty who generously gave their time to ensure the accuracy and quality of the articles in this publication.
- © 2011 MD Conference Express