Summary
This article discussed some of the novel targets for anticoagulants, the two most immediate of which are Factor Xa and Factor IIa (thrombin), as well as overview of the characteristics of five new anticoagulant agents and current studies that involve these agents.
- thrombotic disorders
Jonathan L. Halperin, MD, Mount Sinai School of Medicine, New York, NY, discussed some of the novel targets for anticoagulants, the two most immediate of which are Factor Xa and Factor IIa (thrombin).
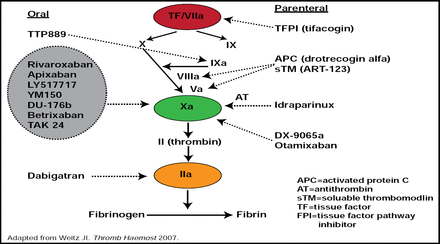
There are several potential targets for novel anticoagulants. “Factor IIa (thrombin) was the first target addressed, because it is the last step in the cascade before the formation of fibrin,” said Dr. Halperin (Figure 1). It also has the advantage of potentially interfering with platelet-thrombin interactions, since thrombin is a potent activator of platelets. These platelet-thrombin interactions may be important not only in acute coronary syndromes (ACS) but also in the mechanism of stroke in atrial fibrillation (AF). Hepatoxicity led to the demise of the first oral thrombin inhibitor (ximelagatran) that was developed; however, an excess in hepatotoxicity was not observed in the clinical trial development with the second agent in this class, dabigatran.
Investigational Anticoagulant Targets.
Reproduced with permission from J. Halperin, MD.
Factor Xa, which acts more proximally in the coagulation cascade than Factor IIa, is also a logical target because of its amplification effect and the potential for a reversible action, which is not available at the level of thrombin. The major safety question for the development of the Factor Xa inhibitors is the potential for bleeding. While there is tremendous promise for excellent efficacy with a very low risk of bleeding with the Factor IX and XI inhibitors, they remain in very early development. Tissue factor (TF/VIIa) is also a very appealing target that is particularly attractive to those who deal with patients in the acute surgical setting, but no trials are currently ongoing in patients with AF or ACS.
Robert P. Giugliano, MD, SM, Brigham & Women's Hospital, Boston, MA, presented an overview of the characteristics of five new anticoagulant agents and current studies that involve these agents (Table 1) and commented on how each might fit the definition of the “ideal anticoagulant” (Table 2).
Trial and Publication Status.
PK/PD Properties of an Ideal Long-Term Oral Anticoagulant.
“Compared with warfarin,” said Dr. Giugliano, “the new anticoagulant therapies have more stable PK/PD profiles, no diet interaction, and fewer drug-drug interactions. They do not require monitoring of their anticoagulant effect and, so far, do not appear to be associated with off-target adverse events.” Dabigatran is the only thrombin inhibitor among the new group. The time to action is short (1 to 4 hours) for those agents for which this information has been reported (all but betrixaban). The only agent that appears to have substantial interaction with the CYP 450 system is rivaroxaban. Bioavailability is good for all of the agents, with the exception of dabigatran, where it is rather low (7%). Protein binding is variable across the group, with rivaroxaban having the highest (>90%). This could be a disadvantage in patients with different levels of plasma proteins, a high state of acute phase reactance, or malnutrition. It could also mean that rivaroxaban is less likely to be successfully removed by dialysis. The half-life of the drugs varies 2-fold or more. Edoxaban has the shortest half-life (8 to 10 hours). Betrixaban has the longest (19 to 20 hours); thus, it would be suitable for once-daily dosing. Renal elimination varies markedly—80% for dabigatran and <5% for betrixaban—which means that there could be roles for more than one agent in this class in clinical practice to accommodate patients with varying renal and hepatic function.
Many excellent alternatives to vitamin K antagonists are on the horizon, and there are meaningful differences in the PK/PD properties of these agents. Trial results so far indicate that dose selection is a critical area for this class of drugs to identify the optimal balance of thrombotic protection and bleeding risk. It also appears advantageous to bring multiple doses forward from Phase II into Phase III testing (rather than the typical single dose that is selected for Phase III) to more thoroughly evaluate these novel agents.
The editors would like to thank the many members of the American College of Cardiology presenting faculty who generously gave their time to ensure the accuracy and quality of the articles in this publication.
- © 2010 MD Conference Express