Summary
This article discusses several approaches to ablative treatment of atrial fibrillation (AF), noting that the primary underpinning of AF ablation is pulmonary vein isolation and that everything else is ancillary, along with advances in imaging during ablation, among other things.
- arrhythmias
- interventional techniques & devices
Douglas L. Packer, MD, Mayo Clinic, Rochester, MN, discussed several approaches to ablative treatment of atrial fibrillation (AF), noting that the primary underpinning of AF ablation is pulmonary vein isolation (PVI) and that everything else is ancillary. Dr. Packer noted, “The key is knowing whether or not you have pulmonary vein isolation—whether you have entrance block or whether you have exit block.” Persistent potentials may remain after ablation, and the question that needs to be answered is whether they are coming from the left superior pulmonary vein or another location, such as the left atrial appendage or the vein of Marshall. One way to answer that question, to avoid overablating, and to confirm entrance or exit block is by pacing at sites that are candidate sources of the potentials and at different locations (eg, laterally or septally) on the vein.
Short- to mid-term clinical success rates using PVI with and without linear ablation in the treatment of paroxysmal AF are quite good (range 80% to 90%); however, in the case of persistent or permanent AF, the success rate within and between techniques varies widely [Brooks AG et al. Heart Rhythm 2010]. Prashanthan Sanders, PhD, University of Adelaide, Adelaide, Australia, discussed whether ablation of AF provided a long-term cure or palliation.
Although there have been only a few long-term AF outcome studies, we have learned several things—most importantly, that although most recurrences are early, there is an attrition rate that is probably related to the underlying substrate, such as hypertension [Shah AN et al. J Cardiovasc Electrophysiol 2008; Sawhney N et al. Am J Cardiol 2009]. This tells us, said Prof. Sanders, that in addition to performing the ablation, clinicians should be aggressively treating the underlying risk factors and monitoring patients over time. At this point, the primary purpose of AF ablation remains symptom control. In order to determine whether ablation can result in a long-term cure, it will be necessary for new studies to clearly delineate both the type of AF and the procedure that was used.
Samuel Asirvatham, MD, Mayo Clinic, Rochester, MN, discussed the complications that are associated with ablation and how they can be avoided. He noted that although the risk of pulmonary vein stenosis can be minimized by not ablating within the pulmonary vein, it still may occur. One of the reasons for this is that there is no anatomical boundary that can be used to identify the beginning of the pulmonary vein and the end of the left atrium. Another reason is that the electrical signals that come from the catheter mapping can be confusing and can lead to overablation. To avoid ablation in the pulmonary vein, it is necessary to have accurate knowledge of the pulmonary vein ostium and to correct for the interpretation of the electrical signals.
Other areas that may be damaged during ablation include the coronary arteries, the aorta, the phrenic nerve, and the esophagus. Catheter entrapment into the mitral valve apparatus is also a recognized complication, which can best be managed by pushing (versus pulling on) the catheter and then reversing the maneuver that caused it to become lodged. In summary, said Dr. Asirvatham, knowledge of the anatomy and an appreciation of its potential complexity and variability is important in avoiding these complications.
David J. Callans, MD, University of Pennsylvania, Philadelphia, PA, discussed some recent and upcoming advances in imaging during ablation. Second-generation image integration takes preprocedure CT or MRI angiograms and incorporates them directly into electroanatomic mapping (EAM) systems. The result is excellent anatomy and the ability to see the patient's unique anatomical variations. This is a strategy that is particularly useful in extrapulmonary vein procedures. Having all of the information in the catheterization laboratory is also very helpful, and one way of doing this is called “fast mapping.” Fast mapping is a way of constructing the atrial geometry within the context of the procedure itself that corrects for the limitations of impedance-based systems. As with most technology, there is a tradeoff, and in this case, it is static images. “3D echocardiography is probably the most perfect 3D imaging in terms of its anatomic correctness,” said Dr. Callans. “However, its tradeoff is that it can not currently be integrated into the EAM environment.” Lastly, he noted that magnetic and robotic navigation promise better catheter stability and a reduction in fluoroscopy exposure for both the operator and the patient.
Looking to the future, Dr. Callans believes that the ultimate imaging for ablation procedures would be direct visual imaging. Two endoscopy-based products that are currently under development (one by Cardiofocus the other by Voyage Medical) would allow clinicians to see in “real time.” A major advantage to real-time imaging is that it makes visual verification of lesion formation possible, which should make gaps in the ablation less likely to occur. Whether by advanced imaging or direct visual imaging, Dr. Callans believes that we need an improved understanding of catheter contact and force and lesion verification. With improved technology, however, will come increased cost and eventually the need for some quantification of effectiveness.
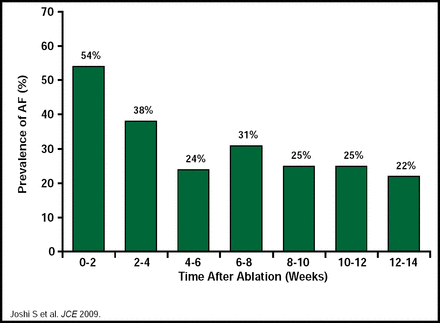
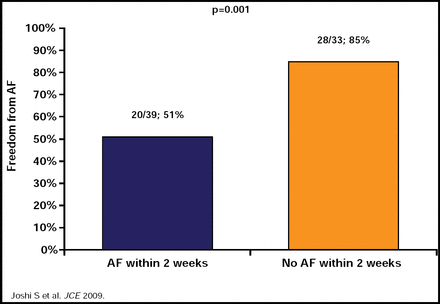
Suneet Mittal, MD, Columbia University, New York, NY, used the current guidelines [Calkins H et al. Heart Rhythm 2007] as the framework for his discussion of the role of monitoring, antiarrhythmic drug (AAD) therapy, and anticoagulation postablation. Current guidelines call for delaying arrhythmia monitoring to assess the efficacy of catheter ablation for at least 3 months postprocedure, called a ‘blanking period,’ because early recurrences of AF are common during that time. Dr. Mittal presented data from a study in 72 patients who received 3 months of continuous ECG monitoring after PVI, which confirmed the early recurrence of AF during the first few weeks after PVI and indicated that a waiting period of 3 months is justified to identify patients with AF recurrences that do not foreshadow procedure failure (Figure 1). In this study, freedom from any AF within the first 2 weeks following ablation was shown to predict long-term AF freedom (Figure 2) [Joshi S et al. J Cardiovasc Electrophysiol 2009].
3-Months Continuous ECG Monitoring Following PVI.
Reproduced with permission from S. Mittal, MD.
3-Month Continuous ECG Monitoring Following PVI: AF Within 2 Weeks Following Ablation.
Reproduced with permission from S. Mittal, MD.
AAD therapy is common during the first 1 to 3 months after ablation. “Although data are limited, probably the best study to evaluate the use of AADs,” said Dr. Mittal, “is the 5A Study.” Results from this study indicated that in patients with paroxysmal AF who were undergoing PVI, 6 weeks of AAD therapy after AF ablation is well tolerated and reduces the incidence of clinically significant atrial arrhythmias and the need for cardioversion/hospitalization for arrhythmia management [Roux JF et al. Circulation 2009]. Longer-term studies are still needed.
Current guidelines call for the use of warfarin for all patients for at least 2 months after ablation, with the decision to extend the use of warfarin beyond this period being based on the patient's risk factors for stroke. Discontinuation of warfarin therapy generally is not recommended in patients with a CHADS score ≥2. Dr. Mittal reviewed the results of several studies that have questioned these recommendations [Bunch TJ et al. J Cardiovasc Electrophysiol 2009; Oral H et al. Circulation 2006; Themistochakis S et al. J Am Coll Cardiol 2010], which demonstrated a low risk of stroke in patients who were undergoing successful AF ablation. “Better stroke risk stratification systems are needed in these patients,” said Dr. Mittal.
- © 2010 MD Conference Express