Summary
This article discusses the complex communication between adipocytes and hepatocytes, noting that understanding this crosstalk is important, since it affects the pathophysiology of type 2 diabetes mellitus. Ultimately one needs to consider three domains: the adipose domain, the mediator domain, and the liver domain.
- Cardiometabolic Disorder
- Obesity
Assaf Rudich, MD, Ben-Gurion University of Negev, Beer-Sheva, Israel, discussed the complex communication between adipocytes and hepatocytes, noting that understanding this crosstalk is important, since it affects the pathophysiology of type 2 diabetes mellitus. Until very recently, when adipocyte-hepatocyte crosstalk was discussed, the focus was on mediators. There is new evidence, however, that indicates that this is a more complex process, and to understand it, we need to consider three domains: the adipose domain, the mediator domain, and the liver domain.
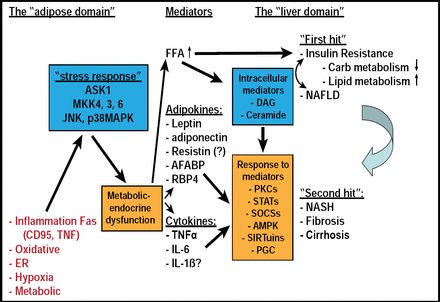
As people become obese, they develop various adipose stresses that are sensed by a specific stress signaling pathway, which, in turn, causes a cascade that translates the stress into a functional alteration of adipose tissue. This alteration causes a change in the mediators (ie, free fatty acids [FFAs], adipokines, cytokines). Hypothesizing on the results of this process, Prof. Rudich noted that it is possible that FFAs participate early on in the natural history of liver disease, in what is sometimes called the “first hit” (ie, insulin resistance and steatosis) and that adipokines, via various adipokine- and cytokine-specific pathways, contribute more to the “second hit,” which is the deterioration of steatosis to nonalcoholic steatohepatitis, fibrosis, and cirrhosis. Having defined this pathway, Prof. Rudich suggested that in order to affect it directly, research should be focused on trying to alleviate the stress of adipose tissue in response to obesity and eliminate the activation of the stress response in adipose tissue (Figure 1).
Role of the Adipocyte in Liver Hepatic Insulin Resistance – From a Paradigm Shift to Paradigm Synthesis.
Reproduced with permission from A. Rudich, MD.
Obesity can impact cardiac remodeling in a variety of ways. Gary Sweeney, PhD, York University, Toronto, Canada, and Institut Pasteur Korea, reviewed some of the ways in which adipokines directly influence the function of cardiomyocytes. He focused on two important but contrasting adipokines: adiponectin, which decreases in obese individuals, and leptin, which increases in obese individuals [Matsuzawa Y. Nat Rev Cardiol 2006; Banerji MA et al. J Clin Endocrinol Metab 1999].
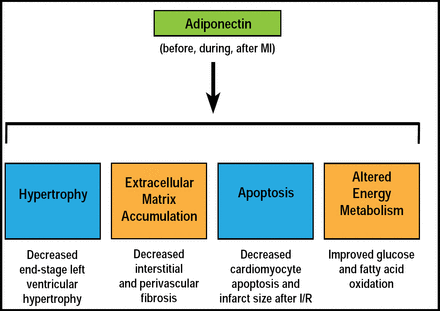
Cardiac remodeling involves various processes that influence the function of the heart, including hypertrophy, apoptosis, fibrosis, and metabolism, all of which are affected by adipokines. Specifically, adiponectin attenuates both myocardial infarction (MI) and angiotension II-induced cardiac hypertrophy [Fuijoka D et al. Am J Physiol Heart Circ Physiol 2006; Wang C et al. Cell Physiol 2010]. In animal studies and in vitro, adiponectin has been shown to have antiapoptotic effects on cardiomyocytes [Wang Y et al. Am J Physiol Endocrinol Metab 2010; Shibata R et al. Nat Med 2005]. Both leptin and adiponectin can affect myocardial matrix remodeling by acting on the cardiomyocytes, fibroblasts, endothelial cells, and smooth muscle cells [Schram K & Sweeney G. Trends in Cardiovasc Med 2008]. Adiponectin has also been shown to protect against MI and angiotensin II-induced cardiac fibrosis, and it can affect metabolism by increasing uptake of both glucose and fatty acid (Figure 2) [Fujita K et al. Arterioscler Thromb Vasc Biol 2008; Palanivel R et al. Cardiovasc Res 2007; Fang X et al. Am J Physiol Endocrinol Metab 2010].
Summary of Effects of Adiponectin on Major Components of Cardiac Remodeling in Heart Failure.
Reproduced with permission from G. Sweeney, PhD.
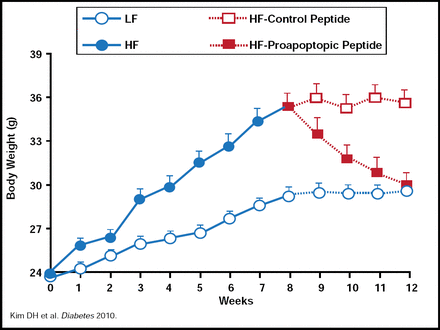
Randy Seeley, PhD, Metabolic Diseases Institute, University of Cincinnati, Cincinnati, Ohio, USA, discussed a new hypothesis, which suggests that disparities in response to high-fat diets (HFDs) may be the result of differences in how quickly individuals can build adipose tissue blood vessels (ie, angiogenesis). Support for this theory comes from research that was originally done at the MD Anderson Cancer Center, showing that targeting a proapoptotic peptide to prohibitin in the adipose vasculature of mice caused ablation of white fat [Kolonin MG et al. Nat Med 2004]. The result was resorption of established white adipose tissue and normalization of metabolism, leading to rapid obesity reversal without detectable adverse effects. Dr. Seeley and colleagues have recently published the results of additional work using this peptide, showing that in mice, treatment with a proapoptotic peptide completely reversed HFD-induced obesity and reduced body weight in those who were on an HFD but not in those who were on a low-fat diet. Fat loss was due to reduced food intake (independent of the action of leptin) rather than a change in energy expenditure (Figure 3) [Kim DH et al. Diabetes 2010]. New, unpublished data from Dr. Seeley's laboratory indicate that glucose metabolism is also improved in mice that are treated with the proapoptotic peptide.
Proapoptopic Peptide Decreases Body Weight.
Reproduced with permission from R. Seeley, PhD.
- © 2010 MD Conference Express