Summary
Results from the NeuroThera® Effectiveness and Safety Trial-2 [NEST-2; NCT00419705] show no difference in efficacy between transcranial laser therapy and sham treatment for the treatment of acute ischemic stroke when applied within 24 hours of diagnosis.
- Ischemia
- Cerebrovascular Disease Clinical Trials
Results from the NeuroThera® Effectiveness and Safety Trial-2 (NEST-2; NCT00419705) show no difference in efficacy between transcranial laser therapy (TLT) and sham treatment for the treatment of acute ischemic stroke (AIS) when applied within 24 hours of diagnosis.
The NeuroThera® Effectiveness and Safety Trial-2 (NEST-2) was a phase III prospective, double-blind, randomized, sham-controlled, multicenter (n=58) study of TLT for the treatment of AIS. The study population included subjects, aged 40 to 90 years, with an NIHSS score of 7 to 22 who were diagnosed with AIS. Evidence of intracranial, subdural, or subarachnoid hemorrhage was cause for exclusion, as were prestroke mRS score ≥3; blood glucose >400 mg/dl or <60 mg/dl; and sustained systolic blood pressure (BP) >190 mm Hg or <80 mm Hg, or diastolic >110 mm Hg or <50 mm Hg. Subjects who had received thrombolytic therapy (tPA) and those with head implant (eg, clipped aneurysm, Hakim valve) also were excluded. Subjects were followed for 90 days.
The primary efficacy endpoint was a modified Rankin Scale (mRS) score of ≤2 at 90 days. Secondary endpoints were the shift in mRS score and NIHSS score 0 or 1 at 90 days or an improvement of ≥9 points. Safety endpoints included mortality, adverse events, and intracerebral hemorrhage.
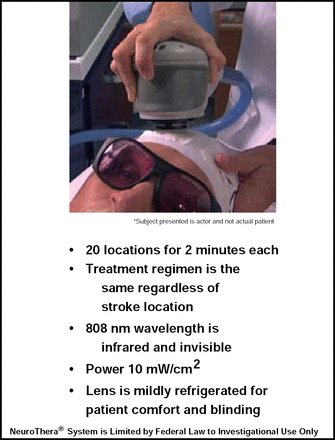
Subjects were randomly assigned to receive TLT (n=331) or sham treatment (n=327). Treatment was performed at 20 locations for 2 minutes each. For patient comfort and to avoid breaking the blind, the lens was mildly refrigerated. Median NIHSS score at baseline was 12 in the treatment group and 13 in the sham group. Mean time to treatment was approximately 14 hours in both groups (Figure 1).
Transcranial Laser Therapy with NeuroThera®.
There was no difference in the primary outcome of mRS score ≤2 (36.3% in the TLT group and 30.9% in the sham group; p=0.094). Adverse events were similar in both groups (Table 1). The shift in mRS score in the treatment group was equal to or better than in the sham population for mRS 0–4; however, there was no difference at the upper end of the score (mRS 5,6).
Safety Results.
TLT is apparently safe and well tolerated, with no important effect of time from onset to treatment within the 24-hour time window. Although there was no difference in efficacy in the overall population, results from a prespecified subset of 434 patients with mRS 7 to 15 showed a significant (p=0.044) treatment effect in 51.6% of TLT patients versus 41.9% of patients in the sham group. “In light of the demonstrated safety of TLT, the results of this post hoc analysis are promising,” said Justin Zivin, MD, San Diego VA Medical Center, San Diego, CA, “as they may indicate a benefit for patients who suffer moderate to moderately severe stroke.” A multinational phase III study is planned (NEST-3).
- © 2009 MD Conference Express