Summary
This article discusses the progress that is being made toward a better understanding of the pathophysiology of pulmonary arterial hypertension (PAH), PAH diagnosis, links between genetics and PAH, as well as combination therapies for this disease, among other things.
- Thrombotic Disorders
- Hypertensive Disease
- Thromboembolic Disease
Marlene Rabinovitch, MD, Stanford University, Stanford, CA, discussed the progress that is being made toward a better understanding of the pathophysiology of pulmonary arterial hypertension (PAH).
Earlier research from Dr. Rabinovitch's laboratory showed that degradation of elastin in PAH leads to increased vascular stiffness and promotes smooth muscle cell proliferation and damage to the distal microcirculation. Using a strain of mice (S100A4) that was susceptible to obstructive neointimal formation in association with perivascular inflammation, she showed that these mice, when infected with human herpes virus (linked to PAH), formed neointimal lesions and heightened production of lung elastin peptides that were associated with invasion of inflammatory cells and intravascular viral antigens [Spiekerkoetter E et al. Am J Physiol Lung Cell Mol Physiol 2008]. Further, the elastic laminae of S100A4 mice were susceptible to degradation by elastase, while the loss of bone morphogenetic protein receptor type II (BMPR2) increased this susceptibility of elastin to elastase. BMPR functions by inhibiting the proliferation of vascular smooth muscle and endothelial cell survival. Genetic alterations of BMPR may lead to degradation of elastin and improve PAH outcomes.
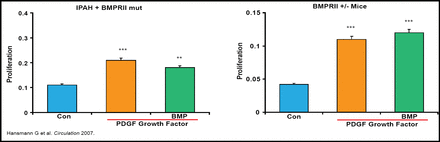
PAH patients also have reduced expression of apolipoprotein E (apoE) and peroxisome proliferator-activated receptor-gamma (PPARγ) in lung tissue, both of which suppress pulmonary artery smooth muscle cell proliferation, which can be reversed by the PPARγagonist rosiglitazone (Figure 1) [Hansmann G et al. Circulation 2007].
The PPARγ Agonist Rosiglitazone Rescues Loss of Growth Inhibition with Loss of BMPRII in PA SMC.
A better understanding of the pathophysiology of PAH offers the potential for the development of more effective treatments, such as elastase inhibitors, PPARγ agonists, and other regulators of pulmonary vascular homeostasis.
While there is strong support for the connection between the genetic mutation of BMPR2 and familial PAH, only 20% of these mutation carriers develop PAH, suggesting that other factors may be involved. James E. Loyd, MD, Vanderbilt University School of Medicine, Nashville, TN, discussed a number of other pathways or modifiers for PAH.
Adult females have an almost 3-fold greater risk for PAH than males. It has been suggested that this gender disparity may be due to the 10-fold expression of the estrogen-metabolizing gene - CYP1B1- that is seen in females without PAH compared with male PAH patients [West J et al. BMC Med Genomics 2008]. In addition, genetic and metabolic markers of altered estrogen metabolism in subjects with a BMPR2 mutation can modify the risk for PAH [Austin E et al. Eur Respir J 2009].
TGF-β induces apoptosis through the SMAD protein pathway. Functional TGF-β 1 SNPs increase TGF-β/BMP signaling imbalances in BMPR2 mutation heterozygotes, leading to PAH diagnoses at younger ages, increased penetrance, and SMAD2 expression in familial PAH [Phillips JA et al. Genet Med 2008].
Expression of the normal BMPR2 allele is decreased in PAH patients, while patients with missense BMPR2 mutations have greater disease severity than those with truncated mutations. In addition, patients with missense mutations are diagnosed with PAH at a significantly younger age and have a shorter survival, from diagnosis to death or lung transplantation, than those with truncated mutations [Austin ED Respir Res 2009].
Another possible modifier that could influence penetrance of familial PAH is the variability in the expression of the wild-type (WT) BMPR2 allele. Unaffected mutation carrier-derived lymphoblastoid (LB) cell lines have higher levels of WT BMPR2 transcripts than familial PAH patient-derived LB cell lines (p≤0.005) [Humid R et al. Hum Mutat 2009].
These new findings suggest that “steady progress is being made in understanding modifiers for clinical expression of BMPR2 mutation and inheritable PAH,” concluded Dr. Loyd.
The diagnosis of PAH is complicated due to overlapping, nonspecific symptoms. Martine Remy-Jardin, MD, University of Lille, France, explained how CT scanning can be used to sort out some of the PAH diagnostic problems. CT is the most frequently used imaging method. Recent significant improvements in this technology allow for the entire thorax to be scanned.
Standard CT angiography that is based on multidetector-row CT (MDCT) technology can aid in the diagnosis and classification of underlying causes of PAH, such as severe chronic obstructive pulmonary disease (COPD), interstitial lung disease, pulmonary veno-occlusive disease, and chronic thromboembolic pulmonary hypertension (CTEPH), and can also provide cardiac functional information—all issues that are indirectly related to PAH. However, standard CT angiography is not an accurate means of assessing PAH itself, due to the lack of systematic relationships between the diameter of pulmonary arteries and PAH.
New CT angiography options are available that can an overcome this problem, based on ECG-gated CT acquisitions of the chest. Nongated MDCT scans can be used to detect high-grade shunts through a patent foramen ovale in patients with severe COPD and unexplained systemic desaturation. Correlations between this method and transesophageal echocardiography demonstrate a high degree of reliability of this technique.
Another recent option is MDCT scanning using two X-ray sources that run simultaneously at different energies, which provides not only high-quality diagnostic scans but also lung perfusion images. Using material decomposition algorithms, it is possible to evaluate the distribution of iodine within lung microcirculation and thus participate in the understanding of the cause as well as consequences of PAH. These new imaging approaches aid in the early recognition, prompt and accurate diagnosis, and identification of the underlying etiology of PAH.
Treatment algorithms for pulmonary hypertension (PH) have changed significantly for the better since the 1998 World Symposium on Primary Pulmonary Hypertension, according to Sean Gaine, MD, University College, Dublin, Ireland. A key outcome of this meeting was the reclassification of primary PH into five new groups, which has led to new therapeutic strategies. PH is now categorized as pulmonary arterial hypertension (PAH); PH due to left heart disease; PH due to lung disease and/or hypoxia; CTEPH; or PH with unclear multifactorial mechanisms.
New discoveries in translational medicine have identified the endothelin, nitric oxide, and prostacyclin pathways as possible treatment avenues. Oral therapies, like bosentan and ambrisentan, are effective endothelin receptor antagonists; sildenafil and tadalafil inhibit phosphodiesterase in the nitric oxide pathway, and the epoproserol, treprostinil, and iloprost prostacyclin analogs act on the prostacyclin pathway. Because of the new treatments, less than 5% of PH patients are now treated with calcium channel blockers, and newer therapies are replacing the use of the standard treatment, epoprostenol.
Improved goals for determining treatment success are also being explored. As patients in NYHA functional class IV at baseline have poorer survival outcomes than patients in functional class III and those in class III have worse outcomes than those in class II or I, treating patients until they reach NYHA I or II has become the desired treatment goal [Sitbon O et al. J Am Coll Cardiol 2002].
Another approach is the use of combinations of bosentan, sildenafil, and inhaled iloprost in conjunction with a goal-oriented treatment strategy. This has been shown to reduce the need for intravenous prostaglandin treatment and lung transplantation [Hoeper MM et al. Eur Respir J 2005]. In some PAH patients, the addition of sildenafil to long-term intravenous epoprostenol therapy improves exercise capacity, hemodynamic measurements, time to clinical worsening, and quality of life [Simonneau G et al. Ann Intern Med 2008].
Using combination therapy, setting and achieving goals that are key to appropriate therapy, getting the correct diagnosis the first time, and obtaining expert referral are essential elements for treating this orphan disease. Referral centers that can increase clinical trial enrollment are also important for developing a better understanding of this disease, concluded Prof. Gaine.
- © 2009 MD Conference Express