Summary
New data challenge the current guidelines for management of hypertension, which recommend initiating treatment with a diuretic and suggest monotherapy as the starting point of treatment. The ACCOMPLISH trial showed that a single-pill combination of an angiotensin-converting enzyme inhibitor and a calcium channel blocker led to excellent blood pressure control and significantly reduced the risk of cardiovascular events in high-risk patients.
- cardiology clinical trials
- hypertensive disease
New data challenge the current guidelines for management of hypertension, which recommend initiating treatment with a diuretic and suggest monotherapy as the starting point of treatment. The ACCOMPLISH trial showed that a single-pill combination of an angiotensin-converting enzyme (ACE) inhibitor and a calcium channel blocker led to excellent blood pressure control and significantly reduced the risk of cardiovascular events in high-risk patients.
ACCOMPLISH was a multinational, double-blind clinical trial that enrolled patients at 550 centers in the US and Nordic countries. Patients were randomly assigned to treatment with a combination of an ACE inhibitor and a calcium channel blocker (benazepril/amlodipine) (5713 patients) or with a combination of the same ACE inhibitor and a thiazide diuretic (benazepril/hydrochlorothiazide) (5733 patients). The starting doses of amlodipine (5 mg), benazepril (20 mg), and hydrochlorothiazide (12.5 mg) were titrated to achieve a blood pressure <140/90 mm Hg or <130/80 mm Hg for patients with diabetes or renal insufficiency. Other antihypertensive agents (eg, beta blockers, alpha blockers, clonidine) could be added to achieve target blood pressure.
The mean age of the patients was approximately 68 years, and about 60% of patients were men. All patients had a systolic blood pressure of at least 160 mm Hg or were already being treated with antihypertensive agents. Before entry in the study, patients had received aggressive medical management, including frequent use of ACE inhibitors/angiotensin II receptor blockers (78%), lipid-lowering agents (67%), and oral antiplatelet therapies (63%).
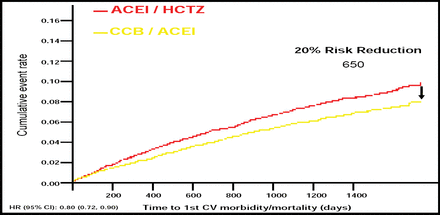
The primary endpoint was cardiovascular morbidity and mortality, defined as cardiovascular-related death, nonfatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina, coronary revascularization procedure, or resuscitated sudden death. Kenneth Jamerson, MD, University of Michigan, Ann Arbor, MI, lead investigator of the study, noted that the trial was stopped early after an interim analysis by the Data Safety and Monitoring Committee demonstrated overwhelming efficacy (crossing of a prespecified efficacy boundary) in favor of benazepril/amlodipine.
Dr. Jamerson reported that at 30 months of follow-up, benazepril/amlodipine (CCB/ACEI) reduced the risk of cardiovascular morbidity and mortality by 20% compared with benazepril/hydrochlorothiazide (CCB/ACEI/HCTZ) (526 events vs 650 events; HR 0.80; 95% CI, 0.72–0.90; p=0.0002). In addition, the individual components of the primary endpoint also favored CCB/ACEI, with the exception of resuscitated sudden death (Figure 1). Blood pressure control improved significantly, from 37.5% to approximately 80% in both treatment groups (p<0.001), and approximately half of the patients in each group needed no antihypertension agents other than the study drugs.
Primary Endpoint.
In the intent-to-treat population in ACCOMPLISH, the composite primary endpoint and its individual components favored CCB/ACEI compared with ACEI/HCTZ. The exception was resuscitated sudden cardiac death. The data shown represent the incidence of adjudicated primary endpoints, based on a cutoff analysis date of March 24, 2008.
“The results of ACCOMPLISH provide compelling evidence for initial combination therapy with an ACE inhibitor and a calcium channel blocker, and these results challenge current diuretic-based guidelines,” said Dr. Jamerson.
- © 2008 MD Conference Express