Summary
Although anti-TNF therapies are established treatments for rheumatoid arthritis (RA), significant proportions of patients do not achieve an adequate response, or become refractory to them. This article presents data from the RADIATE study [NCT00106522], a randomized, double-blind study that investigated the efficacy and safety of treatment with tocilizumab plus methotrexate in patients with moderate to severe RA and a prior history of failed anti-TNF therapy.
- rheumatoid arthritis clinical trials
Although anti-TNF therapies are established treatments for rheumatoid arthritis (RA), significant proportions of patients do not achieve an adequate response, or become refractory to them. Paul Emery, MD, PhD, University of Leeds, Leeds, UK, presented data from the RADIATE study (NCT00106522), a randomized, double-blind study that investigated the efficacy and safety of treatment with tocilizumab (TCZ) plus methotrexate (MTX) in patients with moderate to severe RA and a prior history of failed anti-TNF therapy.
Patients who enrolled in the RADIATE study received placebo (n=158), TCZ 4 mg/kg (n=161), or TCZ 8 mg/kg (n=170) every 4 weeks plus MTX for 24 weeks. The primary study endpoint was ACR20 response; safety and secondary endpoints also were assessed.
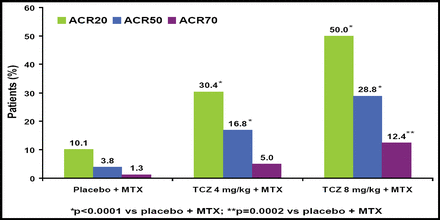
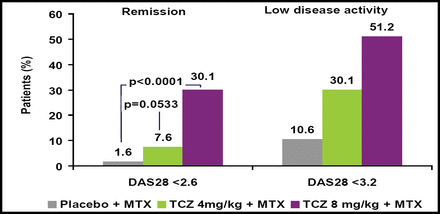
Significantly more patients who received TCZ achieved improvement across all 3 ACR response criteria versus those who received placebo (Figure 1). DAS28 also was significantly improved in TCZ patients versus those who received placebo (Figure 2). TCZ was effective irrespective of the number of or the most recently failed anti-TNF treatments.
Clinical Response at Week 24.
Remission and low disease activity (DAS28) at Week 24.
Both 8 mg/kg and 4 mg/kg doses of TCZ were generally well tolerated. The adverse event (AE) profile was consistent with data from other studies of tocilizumab and with the immunomodulatory properties of the drug. AEs were seen in 81%, 87%, and 84% and serious AEs in 11%, 7%, and 6% of the placebo, 4 mg/kg, and 8 mg/kg groups, respectively. Infections occurred in 41%, 47%, and 50% and serious infections in 3%, 2%, and 5% of the placebo, 4 mg/kg, and 8 mg/kg groups, respectively. There was no difference in safety or tolerability based on prior anti-TNF treatment.
Combination therapy using TCZ plus MTX resulted in clinical improvements in efficacy and safety in this refractory population, irrespective of the number of, or most recently failed, anti-TNF treatments. The dose response favored the 8 mg/kg TCZ dose, and a low proportion of patients in this group experienced serious AEs or serious infections.
Patients who are refractory to one or more courses of anti-TNF therapy have very limited options, so the findings of this trial may have significant impact on clinical management for a select group of patients who are “particularly responsive to IL-6 inhibition,” Prof. Emery said.
- © 2008 MD Conference Express