Summary
Continuous blood glucose monitoring (CGM) is a tool that is used to measure interstitial glucose levels on a continuous basis, as often as every few minutes. CGM allows patients to monitor blood glucose levels as they fluctuate in response to insulin administration, food intake, exercise, and other factors. Ongoing feedback on glucose levels can be helpful for correcting the early signs of hyperglycemia. This article discusses issues that are related to CGM use in pregnant women, for nighttime glucose collection, and in patients with sleep apnea.
- nursing
- sleep disorders
- hyperglycemia/hypoglycemia
- diabetes mellitus
- episodic & paroxysmal disorders
- endocrinology
Continuous blood glucose monitoring (CGM) is a tool that is used to measure interstitial glucose levels on a continuous basis, as often as every few minutes. CGM allows patients to monitor blood glucose levels as they fluctuate in response to insulin administration, food intake, exercise, and other factors. Ongoing feedback on glucose levels can be helpful for correcting the early signs of hyperglycemia. In this session, presenters discussed issues that are related to CGM use in pregnant women, for nighttime glucose collection, and in patients with sleep apnea.
CGM During Pregnancy Improves Maternal and Infant Outcomes
The use of CGM during pregnancy in women with type 1 and type 2 diabetes may have beneficial effects on maternal glycemic control and infant birth weight. In particular, CGM may be beneficial in reducing second and third trimester hyperglycemia that is associated with increased risk of macrosomia, or excessive birth weight among newborn infants.
To evaluate the effects of CGM during pregnancy, Helen R. Murphy, MD, Ipswich Hospital NHS Trust, Ipswich, UK, and colleagues conducted a prospective, open-label trial. In the trial, 71 pregnant women were randomly assigned to standard prenatal care with CGM (n=38) or without CGM (n=33). Among women who were enrolled in the trial, 46 had type 1 diabetes and 25 had type 2 diabetes. All other aspects of prenatal care were identical in the 2 treatment groups.
Use of CGM appeared to improve glycemic control among pregnant women, particularly in the third trimester. Between Weeks 32 and 36 of gestation, women in the CGM group had a lower mean HbA1c (5.8%) than women who did not undergo CGM (6.4%; p=0.007).
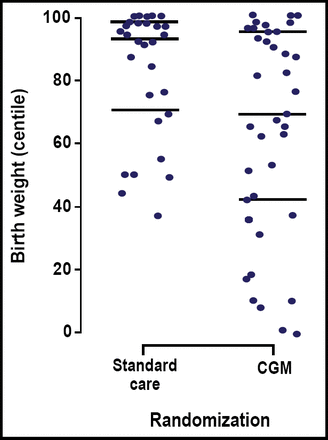
CGM also reduced the risk of excess infant body weight. Infants who were born to mothers in the CGM group had a lower median birth weight percentile compared with those whose mothers did not receive CGM (69% vs 93%; p=0.009). Figure 1 shows the distribution of individual birth weight percentiles among infants who were born in the CGM and standard care-only groups. Overall, CGM lowered the risk of macrosomia by 64% among infants who were born to women in the CGM group (OR=0.36; p=0.05).
Individual Birth Weight Percentiles.
“This is the first trial to demonstrate the effectiveness of CGM on glycemic control and pregnancy outcome,” Dr. Murphy stated. In this study, use of CGM during pregnancy improved glycemic control among women with type 1 and 2 diabetes, lowered infant birth weight, and reduced the risk of macrosomia.
CGM Avoids Stress-Induced Elevations in Blood Glucose Levels
Obtaining nighttime glucose measurements requires sleep disruption and stressful procedures that lead to false estimates. However, researchers do not know the degree to which these procedures alter basic physiology (such as the stress response) and lead to false glucose readings. In this presentation, Christine Berndt, MD, Diabeteszentrum Bad Lauterberg, Bad Lauterberg im Harz, Germany, reported findings of a trial that compared different nighttime glucose measurement techniques.
This prospective study included 30 patients (mean age, 49 years) with type 1 diabetes. On average, patients had been managing type 1 diabetes for 24 years. The mean body mass index (BMI) at baseline was 27.7 kg/m2. The mean daily insulin dose was 43.7 IU, delivered by subcutaneous insulin infusion in 9 patients and by intensified conventional regimens in 21 patients.
Patients were subjected to different methods of nighttime glucose monitoring over 3 consecutive nights. On one night, patients were awakened by alarm clock at 2:00 AM and again at 4:00 AM and instructed to obtain glucose measurements themselves. On another night, an experienced nurse performed capillary blood glucose measurements at 2:00 AM and 4:00 AM, with as little disruption to the sleeping environment as possible (low light, low noise). Glucose measurement techniques (self-measurement with alarm or nurse-assisted) were randomly assigned for Night 1. Patients were left undisturbed on Night 2 and underwent testing with the remaining procedure on Night 3.
All patients also were undergoing CGM with a GlucoDay® device. The CGM device recorded glucose levels between 15 minutes before and 30 minutes after the times that were specified for glucose measurement. The CGM-derived measurements served as reference values and were compared with those that were obtained via self-measurement and by the nursing staff.
Waking in response to an alarm clock significantly increased glucose concentrations, suggesting a stressful arousal. Compared with CGM-derived readings, the mean blood glucose concentrations increased by 15.7 mg/dL after participants were awakened by an alarm at 2:00 AM and by 17.7 mg/dL when participants were awakened by an alarm at 4:00 AM (p=0.0003). After waking in response to an alarm clock, patients also experienced increases in pulse rates and adrenaline concentrations (p<0.05).
By comparison, nighttime glucose measurements that were taken by a nurse under more gentle conditions resulted in only minor elevations in blood glucose. When nurses took blood glucose measurements at 2:00 AM and 4:00 AM, the mean glucose concentrations increased by 4.8 mg/dL and 0.0 mg/dL, respectively, compared with CGM readings. Pulse rates and adrenaline levels were not significantly increased by this procedure.
“Waking up in response to an alarm clock leads to a stressful arousal reaction that will lead to erroneously high glucose concentrations,” Prof. Berndt observed. While hospitalization and gentle blood extraction procedures may provide exact nighttime glucose profiles, this option requires heavy resource use. Therefore, CGM may be a more accurate and cost-effective option for obtaining nighttime glucose profiles.
Sleep Apnea Worsens Overnight Glycemic Control in Type 2 Diabetes
Sleep apnea is a well-defined risk factor for cardiovascular morbidity and mortality, particularly in patients with diabetes. Maria Pallayova, MD, PhD, PJ Safarik University School of Medicine, Kosice, Slovakia, presented data on the glycemic consequences of sleep apnea in patients with type 2 diabetes.
In this trial, Dr. Pallayova and colleagues evaluated nighttime glucose levels in 30 patients who were undergoing CGM. For the analysis, patients were categorized into 4 levels of sleep-disordered breathing (SDB) according to their apnea-hypopnea index (AHI):
-
normal (AHI<5/hour)
-
mild SDB (AHI 5–15/hour)
-
moderate SDB (AHI 15–30/hour)
-
severe sleep apnea (AHI >30/hour)
The presence of sleep apnea appeared to influence nocturnal glucose control. Nighttime glucose profiles were the most stable among normal patients and most labile among patients with severe sleep apnea (p<0.001). There also were greater fluctuations in glucose control among patients with moderate SBD compared with normal patients (p=0.008), and among patients with severe SDB compared with those with mild SDB (p=0.01).
Nocturnal glucose values also were related to mean nocturnal oxygen saturation levels (p<0.001) and minimum nocturnal oxygen saturation levels (p<0.001). In both cases, lower oxygen saturation was associated with higher glucose values.
In summary, Dr. Pallayova and colleagues found that moderate and severe sleep apnea, when accompanied by severe oxygen desaturation, significantly increases overnight glucose variability and worsens nocturnal glucose control in patients with type 2 diabetes. These findings underscore the need to address common diabetes comorbidities, including sleep disturbances.
“With the rapid increase in incidence of coexisting diabetes and sleep apnea, it is important to ensure that patients are treated as effectively as possible in order to achieve sustained glycemic control and, thereby, minimize the impact of the condition,” Dr. Pallayova concluded.
- © 2008 MD Conference Express