Summary
Three new mechanistic approaches to this challenge are highlighted in this article, which Illustrates the systemic complexity of glucose regulation. Included therapies are XOMA 052, TANTALUS, and capsulin.
- insulin
- obesity
- endocrinology
- diabetes mellitus
Illustrating the systemic complexity of glucose regulation, 3 new mechanistic approaches to this challenge were highlighted in the EASD session “New Treatments in Type 2 Diabetes (T2DM).”
XOMA 052
A recent proof-of-concept study has shown that elevated glucose concentrations upregulate the production of the proinflammatory cytokine IL-1β in human pancreatic islet cells (Donath et al. NEJM 2007). These data have been coupled with the observation that there is a concomitant loss of IL-1 receptor antagonist expression in beta-cells of patients with T2DM, which eventually leads to loss of beta-cell function. “These findings implicate an autoinflammatory process in the pathogenesis of type 2 diabetes,” said study author Marc Donath, MD, University Hospital Zurich, Zurich, Switzerland. “This identifies the IL-1β pathway as a target to preserve beta-cell function.”
Prof. Donath reported the interim results of a double-blind, placebo-controlled, dose-escalation study that was performed in the United States and Switzerland to evaluate the safety and pharmacokinetics of XOMA 052 in a cohort of patients with T2DM.
Study participants received a single infusion of XOMA 052, an engineered human anti-IL-1β antibody that targets a dysregulated cytokine pathway in T2DM islet cells, or placebo at 6 dosage levels, from 0.01 to 1.0 mg/kg (n=72; interim analysis, n=48), wherein each subject was evaluated through Day 14 prior to dose escalation. Enrolled patients had to have a duration of disease >6 months, HbA1c levels ≥7.5% and ≤12%, and a BMI of ≥23 and ≤36 kg/m2. Exclusive use of oral glucose-control medications was found in 42.5% of XOMA 052 patients, wherein 32.4% used oral glucose-control medications and injected insulin and 12.5% used insulin only. These treatments were continued during the trial.
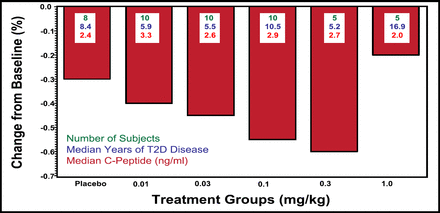
Results were reported at 1 and 3 months. Overall, the study drug was well tolerated, and no hematologic or renal function adverse events were reported. One patient experienced a transient hypoglycemic event. Efficacy at 28 days for all doses of XOMA 052, with the exception of the highest dose (1.0 mg/kg), showed a superior decrease in HbA1c compared with placebo (Figure 1). Insulin release by beta-cells was analyzed at the .01- and .03-mg/kg doses and was increased by 26% after 28 days and by 52% after 91 days. Data for pharmacokinetics indicate that the ∼22-day half-life for XOMA 052 is consistent with monthly or even less frequent dosing.
Prof. Donath stated that the next steps in this drug's development will include finalizing the dose regimen and evaluating XOMA 052′s role in stopping disease progression.
Median Percentage Change in HbA1c at Day 28 With a Single Dose of XOMA 052.
TANTALUS™
Gastric electrical stimulation with TANTALUS™, an implantable pulse generator, is being proposed as an effective electroceutical method by which caloric intake is reduced in obese T2DM patients. Gastric contractility modulation affects the amplitude of antral contractions, thereby increasing the satiety signal outflow from the stomach to the brain. A signal that is amplified through electrical stimulation has been shown to induce satiation in a dog model (Sanmiguel et al. Obesity 2007).
TANTALUS™ is a laparoscopically implanted, subcutaneous, programmable device that has 3 bipolar leads that attach to the gastric wall. The device can be programmed in situ, and the patient recharges the unit once weekly with an external power source.
Data for a study that was concluded in January 2008 were reported by Bruno Guerci, MD, Hopital Jeanne d'Arc, Nancy, France. In this open-label, 24-week investigation, 19 obese patients with type 2 diabetes that was poorly controlled by oral medications were enrolled. Baseline characteristics included an average BMI of 38 kg/m2, HbA1c of 8%, and disease duration of 6 years.
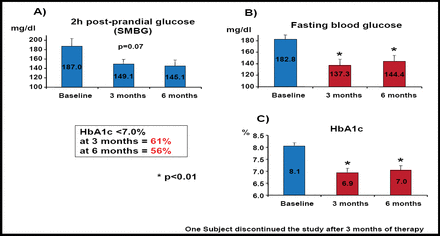
Results of this investigation showed significant reductions in body weight, waist circumference, and systolic and diastolic blood pressures at 6 months (p<0.01). Two-hour postprandial glucose levels were not significantly altered (Figure 2A). However, fasting blood glucose levels were significantly reduced at both 3 and 6 months (p<0.01; Figure 2B). HbA1c levels also were significantly reduced below the target of 7% at 3 months (61% of patients) and 6 months (56% of patients; p<0.01 for both) compared with baseline (Figure 2C). There were 5 nonresponders, who later were determined to have baseline triglyceride levels that greatly exceeded those of responders to electrical stimulus but lower weight, BMI and waist circumference, and HbA1c levels at baseline compared with responders. Across the entire cohort, adverse events were minimal, wherein 4 patients had transient infection around the incision site and 2 patients reported nausea and vomiting.
Biological Efficacy Evaluation.
Capsulin
Capsulin is a formulation of unmodified insulin that is contained in an enteric-coated capsule that protects the active agent from degradation in the stomach and enhances absorption of the drug across the gut wall. This delivery system has the potential to restore glucose homeostasis through hepatic delivery, avoiding peripheral hyperinsulinemia and possibly enhancing patient compliance with chronic treatment. The intended dose is one capsule (150 U) twice daily.
As reported by Timothy Broke-Smith, Diabetology Limited, Cobham, UK, a small phase 2 study of 16 patients was conducted. At study onset, all patients were relatively poorly controlled with oral medications, and at study inception, all medications were withdrawn with the exception of metformin. Patients had a mean HbA1c of 7.4%, BMI of 28.3 kg/m2, and age of 60.2 years.
Over a 10-day period, Capsulin was self-administered by patients one hour prior to both the morning and evening meals. Patients also were responsible for self-glucose monitoring.
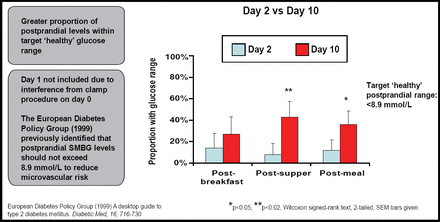
Results showed that daily glucose fluctuations were reduced over the study period, despite 9 of 16 patients having discontinued one or more oral medications. Benefit also was observed in a greater proportion of patients whose postprandial glucose levels fell within the targeted “healthy” range, as identified by the European Diabetes Policy Group (<8.9 mmoL; Figure 3).
Postprandial Glucose Readings.
Significant reductions also were observed for HbAlc, as well as weight and triglyceride levels compared with baseline (p<0.05). No serious adverse events were reported, nor did any patient experience a hypoglycemic episode. No sustained hyperglycemia was observed.
As this novel formulation is further developed, it will be interesting to see if orally administered insulin and the involvement of hepatic glucose management mechanisms generate long-term therapeutic benefits and whether fixed-dose insulin therapy is feasible in patients with poorly controlled T2DM.
- © 2008 MD Conference Express