Summary
Candesartan may have a place in the treatment of retinopathy in patients with type 1 or 2 diabetes, according to findings from the Diabetic Retinopathy Candesartan Trials (DIRECT) study program. The DIRECT-Protect 1 and 2 trials are the first to show the potential benefits of angiotensin-II receptor blockers in patients with baseline diabetic retinopathy.
- endocrinology
- retinal diseases
Candesartan may have a place in the treatment of retinopathy in patients with type 1 or 2 diabetes, according to findings from the Diabetic Retinopathy Candesartan Trials (DIRECT) study program. The DIRECT-Protect 1 and 2 trials are the first to show the potential benefits of angiotensin-II receptor blockers (ARBs) in patients with baseline diabetic retinopathy (Figure 1).
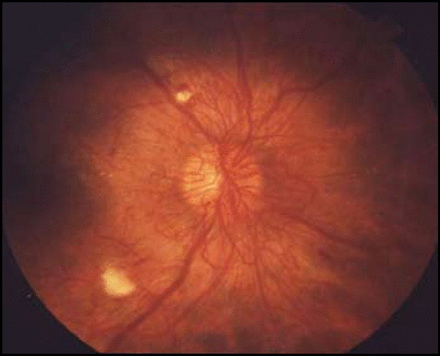
Diabetic Retinopathy.
DIRECT-Protect 1 and 2 examined the effect of candesartan on the progression of retinopathy in patients with type 1 or 2 diabetes, respectively. Nishi Chaturvedi, MD, Imperial College London, UK, presented the findings from DIRECT-Protect 1 and was followed by Anne Katrin Sjølie, MD, Odense University Hospital, Denmark, who presented the findings from DIRECT-Protect 2.
No Benefits in Type 1 Diabetes
The DIRECT-Protect 1 trial included 1905 patients with type 1 diabetes and evidence of retinopathy, which was defined as ≥20/10 up to ≤47/47 on the Early Treatment of Diabetic Retinopathy Study [ETDRS] scale. At baseline, all patients had normal blood pressure (≤130/85 mm Hg) and normal albumin levels. The mean age was 32 years, and the mean duration of disease was 11 years.
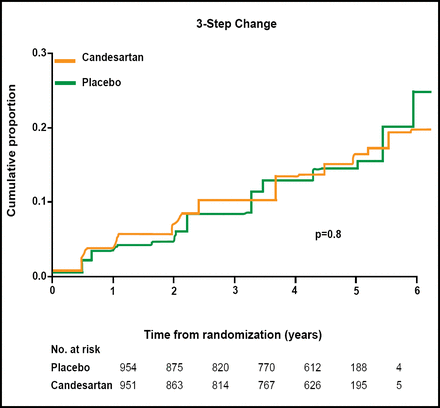
Patients were randomly assigned to treatment with candesartan (n=951) or placebo (n=954) for at least 4 years. The primary endpoint was progression of retinopathy (measured by 3-step change on the 11-point ETDR Scale). By the end of the trial, candesartan appeared to have no effect on retinopathy progression (HR=1.02; p=0.8; Figure 2).
DIRECT-Protect 1: Change in Retinopathy Progression.
Among patients with type 1 diabetes, the most common adverse events (AEs) were nasopharyngitis, hypoglycemia, hypotension, and headache. A similar proportion of patients in the candesartan and placebo groups experienced any AE (77.6% vs 75.8%) or discontinued study medication due to AEs (1.8% vs 1.7%).
Reversal of Retinopathy in Type 2 Diabetes
In the DIRECT-Protect 2 trial, 1905 patients with type 2 diabetes and retinopathy were randomly assigned to treatment with candesartan (n=951) or placebo (n=954). At baseline, 62% of patients had elevated blood pressure (>130/85 mm Hg) and required antihypertensive treatment with an agent other than a RAS inhibitor.
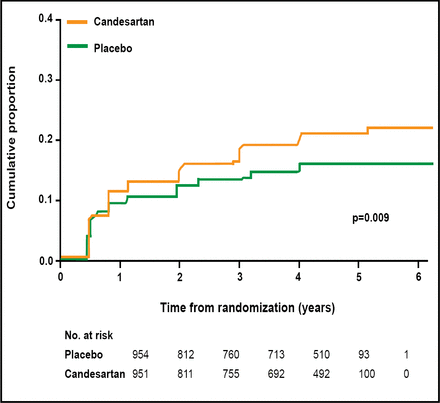
Treatment with candesartan was associated with a nonsignificant 13% reduction in the progression of retinopathy (p=0.2). Therefore, DIRECT-Protect 2 failed to meet its primary endpoint. However, candesartan excelled in a secondary endpoint: regression of retinopathy. Patients in the candesartan group were 34% more likely to experience retinopathy regression (p=0.009) when adjusted for baseline level of retinopathy, diabetes duration, HbA1c level, urinary albumin excretion rate, antihypertensive treatment, and systolic blood pressure during the study (Figure 3). A similar proportion of patients in the candesartan and placebo groups experienced any AE (83.9% vs 82.5%) or discontinued study medication due to AEs (3.9% vs 4.4%).
DIRECT-Protect 2: Retinopathy Regression.
“Diabetic retinopathy is one of the most feared and common complications of diabetes, making this an important clinical finding,” Prof. Sjølie said. “The reversal of this vision-threatening complication of diabetes has not been reported before in large-scale clinical trials.”
The editors would like to thank the many members of the EASD 2008 presenting faculty who generously gave their time to ensure the accuracy and quality of the articles in this publication.
- © 2008 MD Conference Express