Summary
As new bioprostheses become available and implantation techniques are refined, an increasing proportion of patients with valvular disease are avoiding open surgery and undergoing percutaneous procedures. This article describes the advances that are making this possible.
- Interventional Techniques & Devices
- Valvular Disease
Percutaneous Treatments for Valvular Disease
As new bioprostheses become available and implantation techniques are refined, an increasing proportion of patients with valvular disease are avoiding open surgery and undergoing percutaneous procedures. Investigators in this emerging field described the advances that are making this possible.
Percutaneous Aortic Valve Replacement
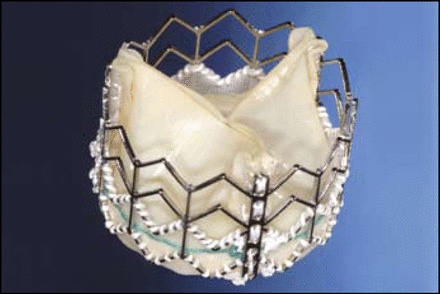
Carlos E. Ruiz, MD, Lenox Hill Hospital, New York, discussed the CoreValve (Figure 1), which uses a self-expanding nitinol cage bioprosthesis made of a porcine pericardial valve. He said most patients are now receiving the 18 French (F) model and not the 25 or 21 F devices on which most observations have been based. The current third-generation model has an over-the-wire design, an 18 F distal end, a 12 F shaft body, a flexible catheter, and dual-speed release handle. “Now, we deploy with no cut-down, no anesthesia, no rapid pacing, and no cardiac support,” Dr. Ruiz noted.
CoreValve.
In 63 patients with advanced heart failure who received the CoreValve, post-PVAR logistic EuroScore was 25.4, including 23.4 in the high-risk group and 31.6 in the nonoperable group. Procedural success was 91%, and 86% of high-risk patients were discharged alive and well with marked hemodynamic and clinical improvement sustained for more than 24 months. Multicenter studies with an 18 F device are ongoing to confirm these results.
E. Murat Tuzcu, MD, of the Cleveland Clinic, described his experience with the balloon-expandable Cribier-Edwards aortic bioprosthesis, which uses a balloon-expandable stainless steel device that is made of an equine pericardial valve and is delivered via an unsheathed catheter (Figure 2). A total of 391 patients received this device between April 2002 and March 2007.
Cribier-Edwards Aortic Bioprosthesis.
In REVIVAL II (Randomization of Endovascular Implantation of Valves), the bioprosthesis was successfully deployed in 87% of patients. Failures were primarily due to access problems that have been improved with re-design. In-hospital mortality was 16.4% (0 to 158 days). The major 30-day complications were iliac artery rupture (9.2%), minor stroke (5.6%), and renal failure (5.5%).
The PARTNER trial will further evaluate this bioprosthesis in 350 high-risk surgical and 250 high-risk inoperable patients.
Percutaneous Pulmonary Valve Replacement
Since percutaneous pulmonary valve replacement was first reported in 2000 it has been shown to improve pulmonary regurgitation, NYHA functional class, exercise tolerance, and other parameters in 158 patients, said Sachin Khambadkone, MD, of Great Ormond Street Hospital, London. The 6-year survival rate is 96%, with freedom from reoperation observed in 83% of patients at a mean of 30 months and in 60% at 63 months.
“We have had some procedural complications—some of which were related to the learning curve—but no deaths,” Dr. Khambadkone said. “New designs have helped prevent device failures.”
Mitral Valve Repair
Clinical proof-of-concept of the percutaneous approach to treating functional mitral regurgitation (MR) via the coronary sinus has been safely demonstrated in humans, reported Steven L. Goldberg, MD, University of Washington Medical Center, Seattle. There is a close relationship between the coronary sinus/great cardiac vein and the posterior annulus of the mitral valve. Several devices have been developed based on the coronary sinus approach by Ample Medical, Cardiac Dimensions, Edwards Lifesciences, Mitralign, and Viacor.
The EVOLUTION (Clinical Evaluation Of the Edwards Lifesciences Percutaneous Mitral Annuloplasty System for the treatment of Mitral Regurgitation) feasibility study of Edwards' MONARC Annuloplasty System showed that 50% of patients achieved a reduction in MR of ≥1 grade at 3 to 6 months. “This was proof that the coronary sinus approach can significantly reduce mitral regurgitation,” Dr. Goldberg said.
Describing the Carillon Mitral Contour System by Cardiac Dimensions, he noted its ability to recapture, which allows the clinician to relieve compromise to the coronary artery as well as deploy a second device. After improvements in design, technical success now exceeds 80%. As each of these systems is modified and patients are more carefully selected, success with the percutaneous approach is growing, he said.
Describing edge-to-edge mitral valve repair, Igor F. Palacios, MD, of Massachusetts General Hospital, Boston, reported that this approach reduces MR to ≤1 in most patients and results are durable up to 36 months. Acute procedural success is now at 90%. Edge-to-edge repair reduces procedure time, produces few complications, and preserves the options for surgery.
In the Phase I EVEREST I trial (Endovascular Valve Edge-to-Edge Repair Study), which evaluated the MitraClip in 55 patients, 76% were discharged with only mild to moderate regurgitation. After 1 year, 75% had avoided surgery and 80% who underwent echocardiography still had only mild-to-moderate regurgitation, or none at all, according to results presented at the American College of Cardiology by Ted Feldman, MD, Evanston Northwestern Healthcare, Evanston, Illinois. “We earlier established that we could reduce mitral regurgitation with the MitraClip, and now we've established the durability of the results,” Dr. Feldman said. EVEREST II is currently enrolling.
- © 2007 MD Conference Express