Summary
Bifeprunox, a partial dopamine agonist, is being developed as a possible treatment for schizophrenia. The results of a 6-month randomized, double-blind, placebo-controlled study of 497 patients with schizophrenia.
- Obesity Clinical Trials
- Schizophrenia
Bifeprunox, a partial dopamine agonist, is being developed as a possible treatment for schizophrenia. The results of a 6-month randomized, double-blind, placebo-controlled study of 497 patients with schizophrenia were presented by Michel Bourin, MD, of the University of Nantes, France.
In order to be included in the study, patients were required to have had a diagnosis of schizophrenia for at least 2 years, a Positive and Negative Syndrome Scale (PANSS) score of at least 60, scores of ≤4 on PANSS items of Hostility and/or Uncooperativeness, and either could not tolerate side effects of their current antipsychotic medications or were experiencing residual symptoms. Patients were randomized to treatment with either bifeprunox 20 mg/day, bifeprunox 30 mg/day, or placebo. Patients were washed off their current medications over a period of 3–6 days. Bifeprunox doses were initiated at 0.25 mg on day 1, and were doubled each day until the target doses of 20 mg or 30 mg were reached. The primary efficacy measure was time to deterioration from the date randomization. Deterioration was defined as one or more of the following criteria: a Clinical Global Impression-Improvement score of ≥5, a PANSS Hostility and/or Uncooperativeness score ≥5 for two consecutive days, or a ≥20% increase in PANSS baseline score.
There were no statistically significant differences between treatment groups in age, gender, baseline weight, or baseline body mass index (BMI).
Both doses of bifeprunox were superior to placebo in the primary efficacy measure, with 41% of the 20 mg group, 38% of the 30 mg group, and 59% of the placebo group reaching deterioration criteria by 6 months (p=0.008 and p=0.006 vs placebo, respectively). The most common adverse events (≥5% and at least double that of placebo) were nausea, vomiting, anorexia, dizziness, dyskinesia, asthenia, and akathisia.
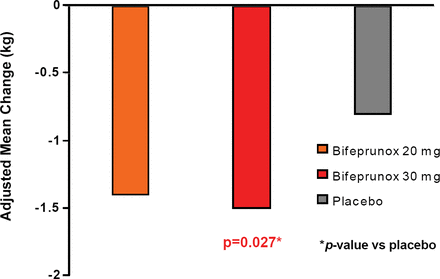
Patients taking bifeprunox 30 mg had a significant decrease in body weight and BMI compared with placebo (−1.5 kg vs −0.8 kg, respectively; p=0.027; Figure 1). Patients in all groups lost weight, regardless of whether or not they experienced adverse events of nausea and/or vomiting, but patients experiencing adverse events of nausea and/or vomiting experienced greater weight loss. Treatment with bifeprunox 30 mg also decreased fasting triglyceride levels compared to placebo (p=0.006); prolactin levels increased in all groups. This compares favorably with many of the currently available antipsychotic medications which are associated with hyperprolactinaemia (Meaney AM et al. Life Sci 2002;71(9):979–92), weight gain, and increases in lipid levels (Newcomer JW. CNS Drugs 2005;19 Suppl 1:1–93). The authors concluded that this agent may be a well-tolerated and efficacious option for stable schizophrenia patients.
Adjusted Mean Weight Change from Baseline to Last Assessment.
- © 2007 MD Conference Express