Summary
Early data on the use of the Edwards SAPIEN transcatheter heart valve (THV) for use in the pulmonary position to treat patients with pulmonic disease show excellent clinical outcomes, as well as the durability of the device without stent fractures or the development of endocarditis. This is according to the results of the first 50 patients enrolled in the Congenital Multicenter Trial of Pulmonic Valve Regurgitation Studying the SAPIEN Interventional THV trial [COMPASSION; NCT00676689].
- cardiology clinical trials
- valvular disease
- interventional techniques & devices
Early data on the use of the Edwards SAPIEN transcatheter heart valve (THV) for use in the pulmonary position to treat patients with pulmonic disease show excellent clinical outcomes, as well as the durability of the device without stent fractures or the development of endocarditis.
Damien Kenny, MB, MD, Rush University Medical Center, Chicago, Illinois, USA, presented updated clinical experience on the use of the SAPIEN valve in the pulmonary position from 2 ongoing trials that are underway in the United States and Europe.
He first presented updated results of the first 50 patients enrolled in the Congenital Multicenter Trial of Pulmonic Valve Regurgitation Studying the SAPIEN Interventional THV trial [COMPASSION; NCT00676689]. This is a prospective, nonrandomized, multicenter study to assess the safety and efficacy of the SAPIEN THV for the treatment of patients with dysfunctional right ventricle– pulmonary artery conduits with or without stenosis.
The primary end point of the trial is freedom from the device or procedure-related death and/or reoperation at 1 year. Secondary end points are freedom from a major adverse cardiac or cerebrovascular event at 6 months, and functional improvement (ie, improved valve function, improvement in exercise tolerance, and freedom from recurrent pulmonary stenosis if treated for stenosis).
Current enrollment in the study is 72 patients from 7 sites in the United States. Of these, 63 have received the valve implant with a median follow-up of 2.33 years, and 55 have been followed for 1 year.
Dr. Kenny presented the characteristics and outcomes of the first 50 patients (Table 1).
Characteristics of the First 50 Patients Treated With SAPIEN THV in the COMPASSION Trial
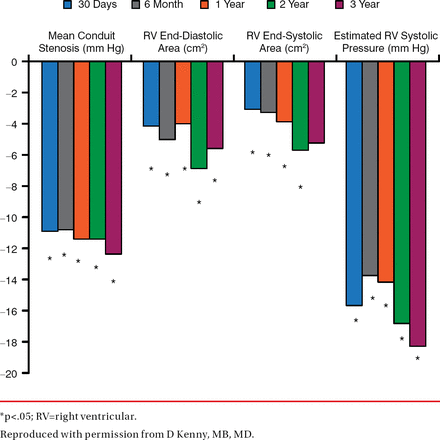
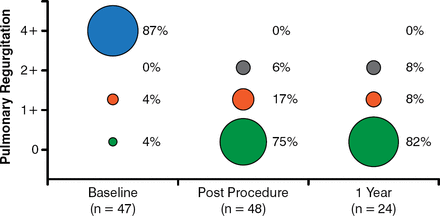
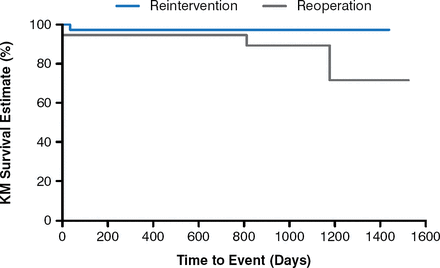
At a follow-up of 87.9 total patient-years, the study found significant echocardiographic changes from baseline (Figure 1), 100% improvement in pulmonary regurgitation at 1 year (Figure 2), and no patients needing re-intervention (Figure 3).
Echocardiographic Changes From Baseline: First 50 Patients in the COMPASSION Trial
*p<.05; RV=right ventricular.
Reproduced with permission from D Kenny, MB, MD.
Pulmonary Regurgitation at 1 Year: 100% Improvement
Reproduced with permission from D Kenny, MB, MD.
Freedom From Reintervention
KM=Kaplan–Meier.
Reproduced with permission from D Kenny, MB, MD.
Similarly, good clinical results were seen in the ongoing European study, the Pulmonic Valve Replacement Multidiscipline EMEA Registry [PREMIER; NCT01356108]. This retrospective-prospective, single-arm, multicenter registry is evaluating the safety and efficacy of the Edwards SAPIEN pulmonic valve for treating patients with conduit failure in the right ventricular outflow tract, or moderate to severe pulmonary regurgitation with or without stenosis. Objectives of the study are to observe the use of the device in routine clinical practice of percutaneous pulmonary valve implantation and to evaluate its efficacy and safety in a real-world situation.
Currently, 131 patients are enrolled in the study, and 130 patients have completed both 6- and 12-month follow-up. Clinical outcomes at 1 year are shown in Table 2.
Clinical Outcomes at 1 Year: PREMIER Registry.
The study also found significant improvements (p < .0001) in pulmonary regurgitation and hemodynamics at both 6 months and 1 year compared to baseline.
Dr. Kenny concluded by stating that in assessing these 2 studies, there is momentum to complete the COMPASSION trial.
- © 2014 MD Conference Express®