Summary
TRO40303 failed to prevent reperfusion injury as measured by creatine kinase and troponin I levels in patients with STEMI when administered just prior to balloon inflation during percutaneous coronary intervention (PCI). This article presents data from the Safety and Efficacy Study of TRO40303 for Reduction of Reperfusion Injury in Patients Undergoing Percutaneous Coronary Intervention for Acute Myocardial Infarction [MITOCARE; Atar D et al. Eur Heart J. 2014].
- Interventional Techniques & Devices
- Cardiology
- Cardiology Clinical Trials
- Myocardial Infarction
- Interventional Techniques & Devices
- Cardiology Clinical Trials
- Myocardial Infarction
TRO40303 failed to prevent reperfusion injury as measured by creatine kinase and troponin I levels in patients with STEMI when administered just prior to balloon inflation during percutaneous coronary intervention (PCI). Dan Atar, MD, Oslo University Hospital Ulleval, Oslo, Norway, presented data from the Safety and Efficacy Study of TRO40303 for Reduction of Reperfusion Injury in Patients Undergoing Percutaneous Coronary Intervention for Acute Myocardial Infarction [MITOCARE; Atar D et al. Eur Heart J. 2014].
TRO40303 blocks the opening of the mitochondrial permeability transition pore and was thought to prevent reperfusion injury. In animal models, TRO40303 reduced infarct size by 50% [Le Lamer S et al. J Transl Med. 2013] and improved left ventricular ejection fraction (LVEF) at 24 hours and 1 month [Augeul L et al. ESC 2009 (poster P4491)]. The purpose of the MITOCARE study was to determine the efficacy and safety of TRO40303 when administered prior to balloon inflation during PCI.
In this multicenter phase 2a trial, 167 patients with STEMI were randomized to either TRO40303 or placebo prior to PCI. The baseline characteristics were similar in both groups, although the TRO40303 arm had a higher mean age (64 vs 60 years) compared with the placebo arm. The mean time from the onset of pain to balloon time was 180 minutes, and the mean door-to-balloon time was 38 minutes.
The primary outcome of the study was infarct size as measured by area under the curve for creatine kinase and troponin I. The secondary outcome was infarct size as measured by magnetic resonance imaging. Patients were eligible for the study if they had no prior STEMI, presented with chest pain within 6 hours, and were to be treated with primary PCI, and there was TIMI 0/1 flow in the culprit artery. Patients were excluded if they had experienced cardiac arrest, ventricular fibrillation, cardiogenic shock, previous coronary artery bypass graft, or atrial fibrillation or they had a pacemaker.
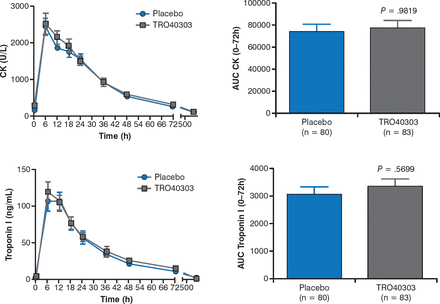
There were no significant differences in creatine kinase (P = .98) or trop onin I (P = .57) levels between the TRO40303 and placebo groups at 72 hours (Figure 1). In addition, the secondary end points—including myocardial salvage index, infarct size, microvascular obstruction, left ventricular end diastolic volume and end systolic volume, and LVEF—were similar between arms.
Effect of TRO40303 on CK and Troponin I Levels
AUC, area under the curve; CK, creatine kinase.
Reproduced with permission from D Atar, MD.
The rate of adverse events was similar between the TRO40303 and placebo arms. However, 25% of patients experienced at least 1 adverse event in the TRO40303 arm, compared with 10% in the placebo arm (P = .01). For example, more patients in the TRO40303 group required repeat revascularization.
In conclusion, Prof Atar stated that the MITOCARE trial showed that TRO40303 does not reduce infarct size in patients with STEMI.
- © 2014 MD Conference Express®