Summary
Experts reviewed current issues in interventional cardiology. This review included the present state of transcatheter valve implantation and repair, the optimal duration of dual antiplatelet therapy after percutaneous coronary intervention, whether or not to treat nonculprit coronary artery disease during the index percutaneous coronary intervention in patients with STEMI, and clinical trials of renal denervation.
- bystander disease
- dual antiplatelet therapy
- interventional techniques & devices
- myocardial infarction
- percutaneous coronary intervention
- renal denervation
- transcatheter aortic valve replacement
Experts at the European Society of Cardiology Congress 2015 discussed recent developments in interventional cardiology, including the state of transcatheter valve interventions, optimal duration of dual antiplatelet therapy (DAPT) following stent placement, treatment options for multivessel disease, and data from the latest renal denervation (RDN) trials.
Transcatheter Valve Interventions
Since the first transcatheter aortic valve replacement (TAVR) procedure in 2002, > 200 000 patients have been treated and multiple valves and delivery systems have been approved for use in clinical care. Alec Vahanian, MD, Paris Diderot University - Paris 7, Paris, France, reviewed the current state of TAVR and transcatheter mitral valve repair and replacement.
Follow-up data indicate that outcomes for high-risk patients with severe aortic stenosis who undergo TAVR are similar to, if not better than, surgical aortic valve replacement [Mack MJ et al. Lancet. 2015]. In addition, complication rates and deaths have continued to decrease over time [Walther T et al. J Am Coll Cardiol. 2015]. Results from the ongoing PARTNER-II [NCT01314313] and SURTAVI trials [NCT01586910] should provide valuable information in the near future.
Available data suggest that transcatheter mitral valve repair using the edge-to-edge technique is safe and improves symptoms in certain high-risk patients. However, additional data from long-term follow-up and randomized trials are needed. Transcatheter mitral valve implantation is feasible, but safety concerns exist and few patients have been treated at this time. There are many challenges facing percutaneous mitral valve replacement, including the positioning, fixation, durability, and retrievability of the valve and developing methods to prevent paravalvular leaks, mitral valve and left ventricular outflow track gradients, and thrombogenicity.
In his concluding remarks, Dr Vahanian emphasized the importance of having an experienced heart team and adequate imaging, as these will help determine the optimal procedure and achieve the best outcomes.
DAPT After Stent Placement
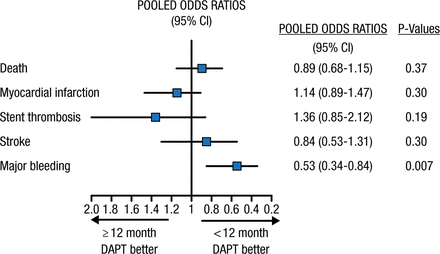
Marco Valgimigli, MD, PhD, Swiss Cardiovascular Center, Bern, Switzerland, reviewed several meta-analyses of trials of DAPT duration after percutaneous coronary intervention (PCI). A meta-analysis of 7 randomized controlled trials indicated that DAPT for < 12 months was associated with a lower risk of bleeding without increasing ischemic risk; however, the power for detecting a significant difference in ischemic outcomes was limited based on the small number of events (Figure 1) [Valgimigli M et al. Eur Heart J. 2015]. Others have reported that for every event of stent thrombosis prevented with prolonged DAPT, about 2.1 events of clinically significant bleeding are expected [Giustino G et al. J Am Coll Cardiol. 2015]. Another meta-analysis of patients with coronary artery disease (CAD) was conducted, in which the analysis was stratified based on DAPT duration in the control group (≤ 12 or > 12 months). Shortening DAPT to < 1 year did not significantly impact mortality (P = .49); however, prolonged DAPT resulted in significantly higher mortality (P = .03) [Navarese EP et al. BMJ. 2015]. Another group conducted a similar meta-analysis using a different statistical approach and found similar results: a 22% increase in mortality with prolonged DAPT (P = .02) [Palmerini T et al. Lancet. 2015]. These results were in contrast to another large meta-analysis that showed no difference in mortality [Mauri et al. Lancet. 2014].
Meta-analysis of 7 Randomized Controlled Trials (n = 15 378)
DAPT, dual antiplatelet therapy.
Reprinted from Valgimigli M, Ariotti S, Costa F. Duration of dual antiplatelet therapy after drug-eluting stent implantation: will we ever reach a consensus? Eur Heart J, 2015, Vol 36, Issue 20, Pages 1219-1222, by permission of the European Society of Cardiology.
In contrast, the recently reported randomized PEGASUS-TIMI 54 trial [Bonaca et al. New Engl J Med. 2015] demonstrated that in > 21 000 stable patients with a history of MI 1 to 3 years prior (including patients without coronary stents), prolonged DAPT significantly reduced cardiovascular (CV) death, MI, and stroke. While this strategy increased major bleeding, it did not increase intracranial hemorrhage (ICH) or fatal bleeding and did not increase mortality. These findings were further supported by a recent subanalysis of a randomized DAPT trial focusing on patients with a history of MI and coronary stenting [Yeh R et al. J Am Coll Cardio. 2015], which showed a reduction in ischemic events without any increase in mortality. Driven by these trials, a meta-analysis presented as a Clinical Trial Update focused on continued DAPT as long-term secondary prevention of ischemic risk in patients with prior MI and found that prolonged DAPT reduced the incidence of ischemic CV events. In particular, prolonged DAPT reduced myocardial infarction, ischemic stroke, and stent thrombosis. Importantly, prolonged DAPT after MI significantly reduced CV death. Prolonged DAPT did increase major bleeding but it did not increase ICH, fatal bleeding, or all-cause mortality [Udell JA et al. Eur Heart J. 2015].
Dr Valgimigli noted that, with more data becoming available, it has become apparent that the focus should shift from protecting the stent to protecting the patient, and that DAPT duration must be individualized. A recent survey of clinicians indicates that this customization occurs in clinical practice, as > 70% of clinicians always account for ischemic and bleeding risk when determining DAPT duration [Valgimigli M et al. EuroIntervention. 2015]. Importantly, as demonstrated by PGASUS-TIMI 54 and the DAPT acute coronary syndrome subgroup, the risk-benefit of prolonged DAPT in patients with prior MI, including those who have never been stented, is more favorable relative to prolonged therapy in patients undergoing coronary stenting for stable disease without prior MI. This may therefore be an important clinical characteristic to guide decision making for clinicians.
Treatment Options for Multivessel Disease
In patients presenting with STEMI, 30% to 50% will have significant CAD in noninfarct arteries. Andreas Baumbach, MD, University of Bristol, Bristol, United Kingdom, reviewed the current evidence regarding treatment of patients with STEMI and multivessel CAD.
The treatment strategy for patients with multivessel CAD in STEMI is controversial. Several trials have been conducted to investigate this issue. The PRAMI trial, which was stopped after an interim analysis, found a clear risk reduction of cardiac death, nonfatal MI, or refractory angina in patients having infarct-artery PCI who underwent nonculprit artery PCI to treat all lesions vs patients who did not (HR, 0.35; 95% CI, 0.21 to 0.58; P < .001). There was no increase in complications in patients who underwent nonculprit PCI [Wald DS et al. New Eng J Med. 2013]. In the CvLPRIT trial, a clear benefit in major adverse CV events was also seen with complete revascularization compared with treating the infarct-related artery only (HR, 0.45; 95% CI, 0.24 to 0.84; P = .009), with no adverse safety findings [Gershlick AH et al. J Am Coll Cardiol. 2015]. In the DANAMI3-PRIMULTI trial, patients who had undergone infarct-related artery PCI were randomly assigned to either no further invasive treatment or fractional flow reserve-guided complete revascularization [Engstrøm T et al. Lancet. 2015]. Results favored complete revascularization (all-cause mortality, nonfatal MI, ischemia-driven revascularization; HR, 0.56; 95% CI, 0.38 to 0.83; P = .004).
Multivessel PCI in STEMI is safer now, because of improvements in devices and thrombotics, and is associated with stabilization of multiple ruptured plaques, avoidance of myocardial stunning of the infarct-related artery (IRA), and hibernation or dysfunction of the non-IRA. It also leads to more rapid improvement and complete revascularization through a single procedure. In contrast, multivessel PCI is also associated with an increased risk of procedural complications, including contrast-induced nephropathy, radiation exposure, and ischemia in the non-IRA. Further considerations in balancing the pros and cons include the heightened state of inflammation and thrombosis with multivessel PCI and overestimation of non-IRA lesion severity.
Dr Baumbach concluded that he feels “we cannot leave bystander disease alone.” Staged PCI with a fractional flow reserve-guided approach appears to be safe during an index admission, but effectiveness of a staged PCI within 2 to 4 weeks of the index event remains uncertain.
Update on RDN Trials
Felix Mahfoud, MD, University Hospital of Saarland, Homburg/Saar, Germany, summarized data from 3 recent randomized controlled trials of RDN. In the DENERHTN trial, patients with uncontrolled hypertension were randomly assigned to RDN plus a standardized stepped-care antihypertensive treatment regimen or the treatment regimen alone [Azizi M et al. Lancet. 2015]. The study met its primary end point, as patients treated with RDN had larger mean reductions in daytime systolic blood pressure (SBP) from baseline to 6 months (P = .03).
In a study sponsored by the University of Leipzig, patients were randomly assigned to RDN or a sham control [Desch et al. Hypertension. 2015]. The intent-to-treat analysis did not show a significant difference between the 2 arms in lowering 24-hour SBP at 6 months (P = .15), but RDN was significantly better in a per-protocol analysis that excluded 6 patients (P = .042).
The Prague-15 trial compared RDN with optimal antihypertensive treatment [Rosa J et al. Hypertension. 2015]. Both arms had a significant decrease in 24-hour SBP from baseline to 6 months (both P < .001), but there was no significant difference between the groups (P = .87). A greater proportion of the patients in the pharmacological group experienced adverse events compared with the RDN group (39% vs 23%, respectively).
In light of the results obtained from the SYMPLICITY HTN-3 trial [Bhatt DL et al. New Engl J Med. 2014], which failed to show a difference in blood pressure control between RDN and a sham control using a blinded design, a panel of experts gathered and published recommendations regarding the design of future clinical trials of RDN [Mahfoud F et al. Eur Heart J. 2015]. This publication offers potential solutions in the areas of medications, patient selection, and procedural considerations as an aid to future research in RDN.
- © 2015 SAGE Publications