Summary
Lung cancers are a heterogeneous group of diseases, and research during the past decade has improved our ability to effectively personalize therapy. This article discusses therapies in oncogene-driven lung cancers, including the emergence of effective targeted therapies in the era of genomics.
- Oncology Genomics
- Cancer
- Respiratory Cancers
- Oncology Genomics
- Cancer
- Oncology
- Respiratory Cancers
Lung cancers are a heterogeneous group of diseases, and research during the past decade has improved our ability to effectively personalize therapy. Rafal Dziadziuszko, MD, PhD, Medical University of Gdansk, Gdansk, Poland, led a series of presentations on local therapies in oncogene-driven lung cancers, including the emergence of effective targeted therapies in the era of genomics.
Until relatively recently, standard treatment for non-small cell lung cancers comprised palliative chemotherapy and radiotherapy, which resulted in poor clinical outcomes, with median survival not exceeding 1 year. With the progression of personalized medicine, however, paradigms for treating lung cancer are evolving. Driver mutations are found in about two-thirds of cases of lung adenocarcinoma, with important predictive and prognostic implications. The identification of an increasing number of driver oncogenes in lung cancers has initiated the development of targeted therapies directed toward those mutations.
In recent years, lung cancer management has therefore evolved toward the stratification of patients on the basis of genetic alterations within driver oncogenes such as anaplastic lymphoma kinase (ALK) and epidermal growth factor receptor (EGFR), each defining a unique molecular subset of lung cancers.
TARGETED THERAPIES IN ADVANCED NON-SMALL CELL LUNG CANCER
Considering the role of targeted agents in multimodality treatment of stage III lung cancers, Solange Peters, MD, PhD, Centre Hospitalier Universitaire Vaudois Centre Pluridisciplinaire d'Oncologie, Lausanne, Switzerland, noted that in the era of positron emission tomography/computed tomography, overall survival of approximately 29 months can be expected for patients with stage III diseases treated with concomitant chemoradiotherapy and targeted agents. However, she stressed that Phase 3 clinical trials to evaluate this combination require much collaborative effort and are difficult to complete.
Patients with ALK-positive lung cancers, for example, may develop early brain metastases and often present with predominantly central nervous system symptoms. Currently, however, the only approved agent for ALK-positive lung cancers, the tyrosine kinase inhibitor (TKI) crizotinib, has poor cerebrospinal fluid penetration. Consequently, central nervous system progression may occur in these patients even in the presence of persistent systemic disease control.
However, a single-arm, open-label, Phases 1 and 2 study was recently undertaken to evaluate the safety and activity of CH5424802, a second-generation ALK inhibitor, in ALK-positive lung cancers. Data showed that patients had excellent systemic and durable responses in the brain, in part because of improved penetration to the brain. Of 46 patients in the Phase 2 portion of the study, 43 (93.5%) achieved an objective response (95% CI, 82.1% to 98.6%). No grade 4 adverse events or deaths were reported, and the study remains ongoing [Seto T et al. Lancet Oncol 2013].
EGFR-TKls were shown to be effective in patients with EGFR mutation-positive lung cancers who present with brain metastases in a Phase 2 open-label study. Patients received either gefitinib or erlotinib once daily, and 23 of 28 participants (83%) showed a partial response. Median progression-free survival and overall survival were 6.6 months (95% CI, 3.8 to 9.3 months) and 15.9 months (95% CI, 7.2 to 24.6 months), with no significant difference between the 2 TKIs [Park SJ et al. Lung Cancer 2012].
LUX-Lung 3 was a randomized, open-label, Phase 3 study of the EGFR-TKI afatinib in patients with lung cancer with asymptomatic brain metastases. First-line afatinib prolonged progression-free survival compared with standard chemotherapy (11.1 vs 6.9 months; HR, 0.58; 95% CI, 0.43 to 0.78; p=0.001) and therefore could be considered a viable treatment option in this patient subset [Sequist LV et al. J Clin Oncol 20l3].
TARGETED THERAPIES IN OLIGOMETASTATIC NON-SMALL CELL LUNG CANCER
Although local therapy is not commonly used in metastatic lung cancers, Helena A. Yu, MD, and Mark G Kris, MD, Memorial Sloan Kettering Cancer Center, New York, New York, USA, reviewed some of the available data, as well as treatment strategies for oligometastatic disease. Under the current paradigm, metastatic disease is considered to be a spectrum of tumor progression, with oligometastatic disease representing an intermediate state of cancer spread between early localized disease and widespread metastases.
Dr. Yu shared study data demonstrating that patients with an oncogene-driver mutation typically respond to oncogene-directed targeted therapy. In a Phase 3, open-label trial comparing gefitinib with carboplatin-paclitaxel chemotherapy in patients with EGFR mutation-positive lung cancers, progression-free survival was increased in patients receiving gefitinib (HR for progression or death, 0.48; 95% CI, 0.36 to 0.64; p<0.001) and decreased for those with EGFR-mutation cancers (HR for progression or death with gefitinib, 2.85; 95% CI, 2.05 to 3.98; p<0.001) [Mok TS et al. N Engl J Med 2009].
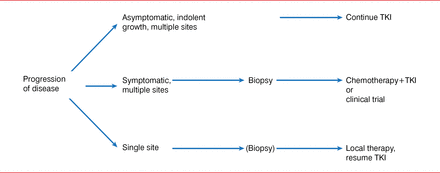
Dr. Kris also stated that local therapy for oligometastatic disease is useful for individuals with acquired resistance to TKI therapy of oncogene-driven lung cancers (Figure 1). He shared results from a study in patients with EGFR-mutation cancers with acquired resistance, in which local therapy was well tolerated, showing that patients can survive a long time with indolent disease. The median time to second progression after local therapy was 10 months (95% CI, 2 to 27 months), and the median time from local therapy until a change in systemic therapy was required was 22 months (95% CI, 6 to 30 months). The median overall survival from local therapy was 41 months (95% CI, 26 to not reached) [Yu HA et al. J Thorac Oncol 2013].
An Algorithm for the Management of Patients With Acquired Resistance to EGFR-TKI Therapy
EGFR=epidermal growth factor receptor; TKI=tyrosine kinase inhibitor.
Reproduced from Yu H et al. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol 203;8(3):346 −51. With permission from Lippincott, Williams & Wilkins/Wolters Kluwer Health.
In summarizing, Dr. Kris indicated that oncogene-driven lung cancers are at the forefront of personalized cancer care, leading the way for the treatment of lung cancers and other solid tumors. Yet although kinase-directed therapies have changed treatment approaches in oncogene-driven lung cancers, they are limited by the development of resistance, and additional research is therefore needed to further improve patient care. Dr. Yu also stressed that improved understanding of the biologic mechanisms of driver mutations will facilitate molecular profiling of subsets of this unique group of cancers, thereby improving the selection of patients for local therapies. She concluded that although patients with EGFR mutation-positive lung cancers represent a small subset of the population of patients with lung cancer, they are an important group to focus on because they have stage IV disease that can potentially be cured, and this is inevitably the ultimate goal of cancer treatment.
- © 2014 MD Conference Express®