Summary
This article presents the joint analysis of 2 randomized comparative trials coordinated by the International Breast Cancer Study Group, the Tamoxifen and Exemestane Trial [TEXT; NCT0006670] and the Suppression of Ovarian Function Trial [SOFT; NCT00066690]. The trials compared adjuvant exemestane plus ovarian function suppression versus tamoxifen plus ovarian function suppression in the treatment of premenopausal women with hormone receptor—positive (HR+) early breast cancer [Pagani O et al. J Clin Oncol 2014].
- Breast Cancer Clinical Trials
- Oncology
Olivia Pagani, MD, presented the joint analysis of 2 randomized comparative trials coordinated by the International Breast Cancer Study Group, the Tamoxifen and Exemestane Trial [TEXT; NCT0006670] and the Suppression of Ovarian Function Trial [SOFT; NCT00066690]. The trials compared adjuvant exemestane plus ovarian function suppression versus tamoxifen plus ovarian function suppression in the treatment of premenopausal women with hormone receptor–positive (HR+) early breast cancer [Pagani O et al. J Clin Oncol 2014].
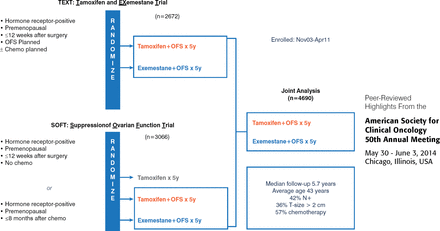
These international trials were designed to address the uncertainty concerning the optimal adjuvant endocrine therapy for premenopausal women with HR+ breast cancer. The current standard of care is the use of tamoxifen for ≥5 years, sometimes supplemented by therapy to suppress ovarian function. The TEXT (n=2762) and SOFT (n=3066) trials were designed to assess if adjuvant therapy with exemestane can improve disease-free survival (DFS) compared with tamoxifen in premenopausal women with HR+ breast cancer also treated to suppress ovarian function (Figure 1).
Design of the TEXT and SOFT Trials
chemo=chemotherapy; OFS=ovarian function suppression; SOFT=Suppression of Ovarian Function Trial; TEXT=Tamoxifen and Exemestane Trial.
Reproduced with permission from O Pagani, MD.
Both trials enrolled premenopausal women with HR+ (estrogen receptor and/or progesterone receptor ≥10%) invasive breast cancer confined to the breast and auxiliary nodes, who had received local-regional treatment with no evidence of residual disease. Randomization to the trial treatments (Figure 2) was within 12 weeks of surgery for all women (TEXT) and women who had not received chemotherapy (SOFT). Women in SOFT who had received (neo)adjuvant chemotherapy were randomized within 8 months of completing chemotherapy, after confirmation of premenopausal status, and could receive oral endocrine therapy before being randomized to combined treatment.
Treatment Regimens in the TEXT and SOFT Trials
GnRH=gonadotropin-releasing hormone; IM=intramuscularly; OFS=ovarian function suppression; q28d=every 28 days; SOFT=Suppression of Ovarian Function Trial; TEXT=Tamoxifen and Exemestane Trial.
Reproduced with permission from O Pagani, MD.
The primary endpoint in both trials was DFS assessed by local, regional, or distant invasive recurrence; invasive contralateral breast cancer; a second invasive malignancy at a site other than the breast; and death by any cause in the absence of the prior occurrence of cancer. Secondary end points included breast cancer–free interval, distant recurrence–free interval, and overall survival.
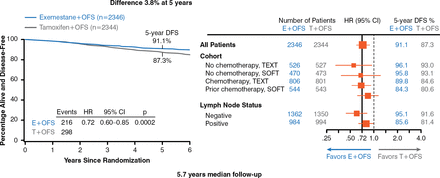
The use of exemestane plus ovarian function suppression achieved the primary end point (Figure 3) and reduced recurrence (Figure 4).
Improvement of DFS by Exemestane Plus Ovarian Function Suppression
DFS=disease-free survival; E=exemestane; OFS=ovarian function suppression; SOFT=Suppression of Ovarian Function Trial; T=tamoxifen; TEXT=Tamoxifen and Exemestane Trial.
Reproduced with permission from O Pagani, MD.
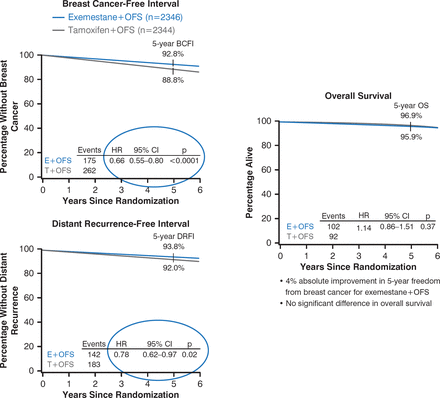
Reduction of Recurrence by Exemestane Plus Ovarian Function Suppression
BCFI=breast cancer–free interval; DRFI=distant recurrence–free interval; E=exemestane; OFS=ovarian function suppression; OS=overall survival; SOFT=Suppression of Ovarian Function Trial; T=tamoxifen, TEXT=Tamoxifen and Exemestane Trial.
Reproduced with permission from O Pagani, MD.
At 5 years, disease-free survival was better with exemestane than with tamoxifen (91.1% vs 87.3%). Overall survival was not significantly different between the 2 groups. Overall, treatment failure occurred in 216 of 2346 patients (9.2%) receiving exemestane plus ovarian function suppression and 298 of 2344 patients (12.7%) receiving tamoxifen plus ovarian function suppression. The initial failure most often (60% of cases) involved distant sites including soft tissue and lymph nodes, bone, and viscera. In women who received chemotherapy, the 5-year absolute improvement in freedom from breast cancer was 5.5% in TEXT and 3.9% in SOFT, and the 5-year freedom from distant recurrence was 2.6% in TEXT and 3.4% in SOFT.
In the subset of women who did not receive chemotherapy (43% of patients) the 5-year absolute improvement in freedom from breast cancer was 3% in TEXT and 2.7% in SOFT, and >97% in the exemestane group remained free from invasive breast cancer at 5 years. Adverse events were generally manageable with medication. Of concern, depression occurred in half the patients in both trials, grade 3 to 4 musculoskeletal events in 11% of those receiving exemestane and 5.2% of those receiving tamoxifen, and osteoporosis was appreciable (39% for exemestane, 25% for tamoxifen).
In summary, if a physician has elected to treat a patient with ovarian suppression, data from SOFT and TEXT suggest that exemestane is superior to tamoxifen. However, it is not yet known if adding ovarian suppression to hormonal therapy is necessary. Data from SOFT comparing tamoxifen alone with ovarian suppression plus tamoxifen and with ovarian suppression with exemestane are expected to be presented at the San Antonio Breast Conference in December 2014.
- © 2014 MD Conference Express®