Summary
This article presents results of the global, Phase 3, randomized Adjuvant Lapatinib and/or Trastuzumab Treatment Optimisation Study; BIG 2–06/N063D [ALTTO; NCT00490139], conducted with more than 8300 women with human epidermal growth factor receptor 2-positive (HER2+) breast cancer. This study evaluated concurrent or sequential adjuvant lapatinib plus trastuzumab. Although the combination is safe, the primary endpoint of improved disease-free survival was not met.
- Breast Cancer Clinical Trials
- Oncology
- Breast Cancer
- Oncology Clinical Trials
Martine J. Piccart-Gebhart, MD, PhD, Universite Libre de Bruxelles, Brussels, Belgium, presented results of the global, Phase 3, randomized Adjuvant Lapatinib and/or Trastuzumab Treatment Optimisation Study; BIG 2–06/N063D [ALTTO; NCT00490139], conducted with more than 8300 women with human epidermal growth factor receptor 2-positive (HER2+) breast cancer. This study evaluated concurrent or sequential adjuvant lapatinib plus trastuzumab. Although the combination is safe, the primary endpoint of improved disease-free survival (DFS) was not met.
ALTTO was prompted by findings from smaller trials of the superiority in the neoadjuvant setting of the lapatinib plus trastuzumab dual blockade of HER2 compared with trastuzumab alone in achieving a pathologic complete response (pCR). In total, 8381 HER2+ breast cancer patients with newly diagnosed early-stage disease were recruited from 44 countries. Following surgery, the women were randomly assigned to receive one of four regimens: trastuzumab alone, lapatinib alone, trastuzumab followed by lapatinib, or trastuzumab plus lapatinib for 12 months. All patients received adjuvant chemotherapy given either before or concomitantly with anti-HER2 therapy. All regimens involved chemotherapy as a backbone. Patients with hormone-positive breast cancer also received hormonal therapies. The arm utilizing therapy with lapatinib alone (n=2097) was stopped early because of futility.
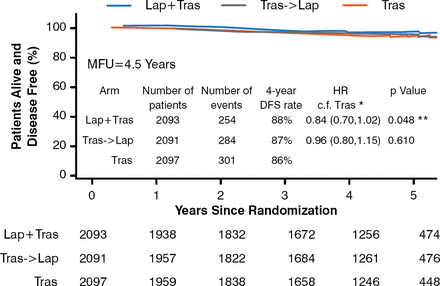
The primary endpoint was DFS as indicated by recurrence of invasive breast cancer at any site, a second primary cancer, or death from any cause. Patients were followed for a median of 4.5 years. In 6281 patients, DFS at 4 years was 88% in the lapatinib plus trastuzumab arm and 86% in the trastuzumab arm (HR, 0.84; 97.5% CI, 0.70–1.02; p=0.048); the differences in DFS between the groups was not significant, as significance was set at p≤0.025. The 4-year DFS rate for trastuzumab followed by lapatinib was 87%, but this was also not significant when compared with trastuzumab alone (HR, 0.96; 97.5% CI, 0.80–1.15; p=0.610; Figure 1).
Disease-Free Survival
DFS=disease-free survival; Lap=lapatinib; MFU=median follow-up; Tras=trastuzumab. *97.5% CI; **p≤0.05 required for statistical significance.
Reproduced with permission from MJ Piccart-Gebhart, MD, PhD.
Statistically similar (and high) DFS was also evident in hormone receptor-positive and -negative women. OS in the 3 trial arms was statistically indistinguishable (Figure 2).
Overall Survival
DFS=disease-free survival; Lap=lapatinib; MFU=median follow-up; Tras=trastuzumab. *95% CI.
Reproduced with permission from MJ Piccart-Gebhart, MD, PhD.
In 6213 patients, concurrent lapatinib and trastuzumab (L+T) was associated with more frequent adverse events compared with trastuzumab alone: diarrhea, 75% versus 20%; rash or erythema, 55% versus 20%; and hepatobiliary, 23% versus 16%. The respective rates for the sequential trastuzumab-lapatinib arm (T→L) were 50%, 24%, and 49%. The rates of any cardiac event were 3.7% for the concurrent lapatinib arm, 2.4% for the sequential lapatinib arm, and 4.5% for the trastuzumab alone arm. Corresponding rates of primary cardiac events were 0.97% (L+T), 0.25% (T→L) and 0.86% (T alone).
These findings suggest that adding lapatinib to adjuvant trastuzumab therapy does not provide any benefit. The results of the NeoALTTO study suggested that this dual anti-HER2 strategy led to a higher pathologic complete response (pCR) rate than did trastuzumab alone; however, the findings of the ALTTO study did not demonstrate that the combination is more effective. A possible reason for the discrepancy is that part of the chemotherapy (the anthracycline component) was given after surgery in some of the neoadjuvant trials. Caution is needed in evaluating new agents for their “potential” based on pCR, and the results of preoperative studies may not predict the results of adjuvant trials. These obstacles may have implications in the accelerated approval of new agents put forth by the United States Food and Drug Administration, for which it has been suggested that pCR may represent an adequate surrogate to adjuvant results.
- © 2014 MD Conference Express®