Summary
This article discusses the results of the Androgen Ablation Therapy With or Without Chemotherapy in Treating Patients With Metastatic Prostate Cancer trial [CHAARTED; NCT00309985]. The trial demonstrated that the use of docetaxel at the beginning of androgen deprivation therapy (ADT) increases overall survival (OS) in patients with metastatic prostate cancer by >1 year compared with ADT alone.
- Reproductive Cancers
- Oncology Clinical Trials
- Reproductive Cancers
- Oncology
- Oncology Clinical Trials
Christopher Sweeney, MBBS, Dana-Farber Cancer Institute, Boston, Massachusetts, USA, described the results of the Androgen Ablation Therapy With or Without Chemotherapy in Treating Patients With Metastatic Prostate Cancer trial [CHAARTED; NCT00309985]. The trial demonstrated that the use of docetaxel at the beginning of androgen deprivation therapy (ADT) increases overall survival (OS) in patients with metastatic prostate cancer by >1 year compared with ADT alone.
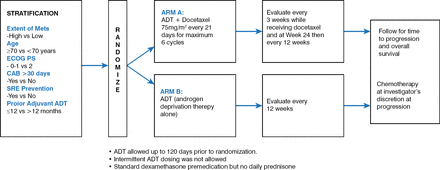
The knowledge that ADT can limit prostate cancer dates back to the 1940s [Huggins C et al. Cancer Res 1941]. More recently, the benefit of docetaxel in improving OS in men with metastatic, castration-resistant prostate cancer was demonstrated [Tannock IF et al. N Engl J Med 2004]. Nonetheless, the combination of early chemotherapy and ADT is debatable. The randomized Phase 3 CHAARTED trial was undertaken to address this debate. The hypothesis of the trial was that docetaxel added at the start of ADT in men with hormone-naïve metastatic prostate cancer would prolong OS. The design of the trial is shown in Figure 1.
Design of the CHAARTED Trial
ADT=androgen deprivation therapy; ECOG PS=Eastern Cooperative Oncology Group performance status.
Reproduced with permission from C. J. Sweeney, MBBS.
The key eligibility criteria were the presence of metastatic prostate cancer, ADT limited to 120 days before study randomization or to adjuvant treatment within the prior 24 months with no disease progression within 12 months after completion of the treatment, Eastern Cooperative Oncology Group performance status of 0 to 2 (the latter only if due to prostate cancer), functions of select organs judged suitable for docetaxel application, and no previous use of docetaxel.
A total of 790 men (89% Caucasian; median age, 63 years; range, 36 to 91 years) newly diagnosed with hormone-sensitive metastatic prostate cancer were recruited from June 2006 to November 2012 and randomly assigned to ADT plus docetaxel (n=397) or standard ADT alone (n=393). Docetaxel was given as a 75 mg/m2 dose every 3 weeks for 6 cycles within 4 months of starting ADT.
Both arms were similar at baseline. Patients were stratified with respect to high-volume (visceral metastases and/or ≥4 bone metastases) or low-volume disease, androgen suppression >30 days, age, Eastern Cooperative Oncology Group performance status 0 or 1 versus 2, prior adjuvant ADT, and the use of an approved drug to delay skeletal-related events. Radiotherapy and prostatectomy had each been performed in 24% of the subjects.
The primary endpoint was OS. Secondary endpoints included the rate of prostate-specific antigen level <0.2 ng/mL at 6 and 12 months, time to progressive disease (biochemical, radiographic, or symptomatic), adverse events and drug tolerability, and quality of life up to 12 months following randomization.
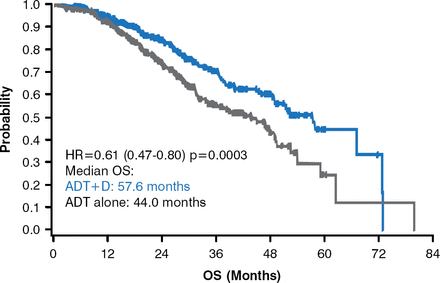
The primary endpoint of OS was met. The median OS of the ADT-plus-docetaxel arm was 57.6 months compared with 44.0 months for men who received ADT only (HR, 0.61; 95% CI, 0.47 to 0.80; p=0.0003; Figure 2).
Overall Survival
ADT=androgen deprivation therapy; D=docetaxel; OS=overall survival.
Reproduced with permission from C. J. Sweeney, MBBS.
The 13-month difference translated to a 39% reduction in the survival hazard (HR, 0.61; 95% CI, 0.47 to 0.80; p=0.0003). Those with a high disease burden at the time of enrollment displayed an even more pronounced survival difference, with median OS of 49.2 months for ADT plus docetaxel treatment compared with 32.2 months for ADT alone (HR, 0.60; 95% CI, 0.45 to 0.81; p=0.0004). The secondary outcomes significantly favored ADT plus docetaxel treatment: prostate-specific antigen response (<0.2 ng/dL at 6 months: 27.5% vs 14.0% [p<0.0001]; <0.2 ng/mL at 12 months: 22.7% vs 11.7% [p<0.0001]), time to castration resistance (20.7 vs 14.7 months, p<0.0001), and time to clinical progression (32.7 vs 19.8 months, p<0.0001).
The trial was ended prematurely when a planned interim analysis revealed the compelling statistically significant survival difference between the treatment arms.
As of mid-January 2013, at a median follow-up of 29 months, there had been 237 deaths: 101 in the ADT-plus-docetaxel arm, of which 84 (83.2%) were due to prostate cancer, and 136 in the ADT-alone arm, of which 112 (83.6%) were due to prostate cancer. The adverse events in the patients treated with docetaxel were generally mild and were manageable. There were no Grade 5 adverse events, although there were 9 cases of Grade 4 neutropenia.
The data demonstrated that ADT and 6 cycles of docetaxel significantly improved OS compared with ADT alone in men with hormone-sensitive prostate cancer. The results are likely practice changing.
- © 2014 MD Conference Express®