Summary
There have been considerable advances in breast cancer treatment over the last 30 years, but radiation therapies will continue to be important in treatment. This article presents an overview of changes and constants in treatment over the past 30 years, including the role of clinical trials and perspectives on the possibilities for the future.
- Radiation Therapy
- Breast Cancer
- Radiology
- Radiation Therapy
- Oncology
- Breast Cancer
- Radiology
There have been considerable advances in breast cancer treatment over the last 30 years, but radiation therapies will continue to be important in treatment. Bruce Haffty, MD, Rutgers Cancer Institute of New Jersey, Robert Wood Johnson Medical School, and New Jersey Medical School, New Brunswick, New Jersey, USA, used his presidential address to present an overview of changes and constants in treatment over the past 30 years, including the role of clinical trials and perspectives on the possibilities for the future.
In 1985, the first National Institutes of Health consensus guidelines for breast cancer treatment specified that premenopausal women with positive nodes should receive chemotherapy, but they did not recommend chemotherapy as a general treatment for other groups of women. Instead, radiation therapy following mastectomy was the standard of care. A randomized trial involving > 900 women (premenopausal and postmenopausal) with high-risk breast cancer showed no significant difference in progression-free survival [Rutqvist LE et al. Int J Radiat Oncol Biol Phys. 1989]. However, postmenopausal women had significantly lower local-regional relapse and distant metastasis when treated with postmastectomy radiation therapy (PMRT). In node-positive postmenopausal women, PMRT has shown superior results. However, the merits of PMRT continue to be debated and have been revisited. Recent studies, such as the EBCTCG Meta-Analysis, MA.20, and EORTC 22925, continue to examine this issue.
Other aspects of diagnosis and treatment have changed since 1984, when mammography was less common. Dr Haffty first focused on ductal carcinoma in situ (DCIS), a relatively uncommon diagnosis (5% of breast cancers) at the time. Mastectomy was the standard treatment; hormonal therapy and tamoxifen were not used; and treatment decisions did not involve testing for estrogen receptors, Her2/neu, or other molecular markers. In contrast, DCIS now represents > 20% to 30% of breast cancers (often detected through mammography). Lumpectomy with radiation therapy is the preferred therapy; tamoxifen or hormonal therapy is commonly used for estrogen receptor-positive cancers; and gene profiling is common. A trial is currently underway to determine whether Herceptin is an effective adjuvant treatment for these cancers. Despite these advances, Dr Haffty noted that the debate over mammography guidelines could lead to reduced early detection of DCIS, which is highly curable, and to increased diagnosis of invasive cancers.
In the future, detection may be improved (with even earlier diagnosis) through techniques such as FAST MRI and tomosynthesis (3D mammography); molecular profiling may play a larger role in determining the best treatment for a specific cancer; and immunotherapy may be effective in preventing and treating DCIS. Tomosynthesis has already been found to lower recall rate and to improve cancer detection rate [Friedwald SM et al. JAMA. 2014], while FAST MRI has been demonstrated to increase cancer yield when compared with mammography [Kuhl CK et al. J Clin Oncol. 2014]. A phase 1 trial has already been completed showing potential for a dendritic cell vaccine, while a randomized trial has been planned pending funding. Eventually, it may be possible to combine radiation therapy with a single dose of a vaccine.
As with DCIS, approaches to treating early-stage invasive breast cancer have changed since 1984. At that time, mastectomy was the preferred treatment. When breast-conserving surgery with radiation was used, brachytherapy was also commonly used. Both protracted radiation schemes and hypofractionation were used. Thirty years later, lumpectomy with radiation is preferred to mastectomy; radiation is often targeted rather than administered to the entire breast; and radiation is not used for certain subsets of patients. Recently, there has been another increase in mastectomy rates, an increased use of brachytherapy, and an increased use of hypofractionation. In the future, more information about specific tumors may allow radiation to be avoided by some patients; lumpectomy may be replaced by radiofrequency ablation and radiosurgery; and hypofractionation may become more aggressive. Additional gene profiling is already becoming routine, being related to local-regional outcomes and being tested in trials. New, more aggressive hypofractionation regimens are also in trials.
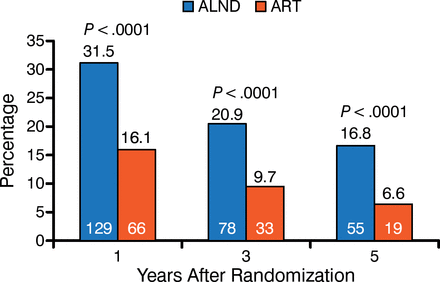
Regional nodal management differs on the basis of whether the node is clinically negative, pathologically positive, or clinically positive. In 1984, extensive axillary dissections were routine, even though a study had demonstrated that axillary radiation was as effective for patients in the clinically negative category. Thirty years later, axillary dissections are less common, and sentinel node samplings are used. If the sentinel node sampling is negative, axillary radiation is not used. Recent studies, including AMAROS, suggest that axillary radiation is not always needed even if sentinel node sampling is positive. The AMAROS trial showed that relapse rates were low with both dissection and with radiation, but that lymphedema and quality of life were higher in the radiation arm (Figure 1) [Rutgers EJ et al. J Clin Oncol. 2013]. As a result, axillary radiation is considered an appropriate treatment for patients that are sentinel node positive. In the future, a combination of improved molecular techniques and imaging will improve decision making so that patients receive additional treatment only if needed.
AMAROS Trial Data Showing Lymphedema
ALND, axillary node lymph dissection arm (blue); ART, axillary radiation therapy arm (gray). Years after randomization is displayed on the x-axis, and percentage of patients is represented on the y-axis.
Source: Rutgers EJ et al. J Clin Oncol. 2013.
In 1984, patients with pathologically positive nodes were treated with extensive full dissection of nodes combined with extensive nodal irradiation that often had significant effects on surrounding tissues. Patients who had negative nodes but were at high risk were also often treated with regional irradiation. In 2014, dissection is used less frequently; some patients are observed rather than treated with radiation; irradiation is more targeted; and nontarget organs are subject to less radiation. Evidence from recent studies (including MA.20 and EORTC) suggest that regional node irradiation is helpful for patients with fewer positive nodes or even with negative nodes but high risk. It is important to continue to develop better approaches to identify subgroups of patients that will benefit, while reducing risks to maximize benefits versus costs. A randomized trial has been proposed to determine whether proton treatment would be clinically beneficial.
Finally, patients with clinically positive nodes in 1984 were generally treated with surgery prior to any radiation therapy unless the cancer was inoperable, extensive nodal dissection was used, and radical mastectomy followed by radiation was the only option for a local-regional curative treatment. In 2014, systemic treatment is routinely used prior to surgery and is often effective. Breast-conserving surgery followed by radiation is commonly used, rather than radical mastectomy, for patients who respond well to systemic treatment. Axillary or sentinel node sampling may be used after systemic treatment. In the future, patients who respond well may not need radiation therapy, and complete nodal dissections may be less frequently used. The Alliance trial is already ongoing to compare dissection with regional nodal irradiation in node-positive patients and in patients who became node negative after initial treatment.
In conclusion, Dr Haffty emphasized that local-regional disease control is important and that radiation therapy is still important in most cases because of microscopic, subclinical, local-regional disease. Dr Haffty explained that radiation therapy will continue to be important, taking a larger local role as systemic therapies improve, but it is important to continue to consider alternative strategies to avoid unnecessary use of radiation therapy.
- © 2014 MD Conference Express®