Summary
Despite the dangers associated with increased resistance, the rate of new antibiotic development has been decreasing over the last 30 years. This article reviews the recent multipronged efforts to address these issues via support for research and development, enhancement of regulatory pathway options that permit progression via smaller programs, and development of innovative options for antibiotic business models.
- Bacterial Infections
- Screening & Prevention
- Drug Resistance
- Infectious Disease
- Bacterial Infections
- Exclusive Article - For home page
- Screening & Prevention
- Drug Resistance
Effective antibiotics are what make modern medicine possible. Unlike many other therapies that only abate symptoms, antibiotics are curative. Without them, our treatment of heart disease, premature birth, and cancer would be much less successful. In some ways, however, their power made things too easy (why provide fresh water or vaccinate when the infection can be treated?) and led to overuse. Although we are learning to be better stewards of the drugs that we do have, we must remember that every use of an antibiotic—whether correct or incorrect—leads to resistance, and this threatens public health worldwide.
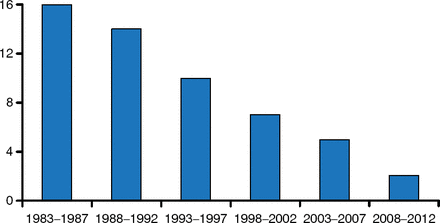
Despite the dangers associated with increased resistance, the rate of new antibiotic development has been decreasing over the last 30 years (Figure 1). It is hard to discover and develop new antibiotics, and often the economic returns do not justify the cost of development. Fixing this will require us to see the problem as an ecosystem. In this keynote address, John H. Rex, MD, AstraZeneca Pharmaceuticals, Waltham, Massachusetts, United States, reviewed the recent multipronged efforts to address these issues via support for research and development, enhancement of regulatory pathway options that permit progression via smaller programs, and development of innovative options for antibiotic business models.
Number of New Antibiotics Developed Over the Past 30 Years
Reprinted from Clin Infect Dis, Boucher HW et al, 10 × 20 Progress—Development of New Drugs Active Against Gram-Negative Bacilli: An Update From the Infectious Diseases Society of America, 2013;56(12):1685–1694, Copyright 2013, with permission from Infectious Diseases Society of America.
Although it is easy to find targets and agents to attack these bacteria, it is difficult to find antibiotics that kill bacteria and are safe. The dose of an antibiotic needed to eradicate bacteria is 20 to 100 times higher than the dose needed for other treatments (eg, typical cholesterol medications range from 5 to 20 mg/d while antibiotics range from 100 to 200 mg/d). Antibiotics are also chemically different. Typical corporate drug libraries contain drugs that have very different properties than antibiotics—that is, higher logD values (measure of water solubility at a given pH) and lower molecular weight.
Research protocols for most new drugs use a superiority design, but this does not work for antibiotics. In almost all cases, it is unethical to randomize patients with resistant pathogens to potentially ineffective and/or toxic therapy. In a situation where a nephrotoxic drug is justified as standard therapy for highly resistant pathogens, a superiority-based approach might be justified but not offer a long-term path to a diverse, vibrant antibiotic pipeline. We need to make the non-inferiority trial design work, but such designs are harder to use.
The totality of the data is unusually strong for antibiotic use. Antibiotics work on an easily isolated microbe, not the person. It is also easy to interpret efficacy data, as results from in vitro and animal studies reliably predict efficacy. Furthermore, unlike with most other drugs, pharmacokinetic/pharmacodynamic data provide direct proof of causality and reduce the need for empirical causality validation via multiple trials.
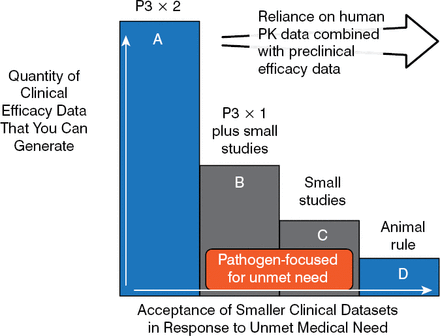
Using this logical framework, Dr Rex proposed a tiered regulatory framework that allows either disease- or pathogen-based label research designs. These smaller pathogen-focused trial designs (B and C in Figure 2) can be used to fill the gap between large, phase 3, noninferiority studies and animal trials that rely on pharmacokinetic/pharmacodynamic data [Rex JH et al. Lancet Infect Dis. 2013] (Figure 2).
Trial Designs Using Pathogen-Focused Pathways
Reprinted from Lancet Infect Dis, 13, Rex JH et al, A comprehensive regulatory framework to address the unmet need for new antibacterial treatments, 269–75, Copyright 2013, with permission from Elsevier.
Thus, a phase 3 program can be based on a tier B design only—drug X versus a standard comparator at one body site—while a resistant pathogen study might be based on drug X versus best available therapy for highly resistant pathogens at multiple body sites. A tier C design would be used in the case of narrow-spectrum agents that cover one of many possible pathogens in a syndrome. In 2013, these design ideas were incorporated into the antibacterial guidelines of both the European Medicines Agency and the US Food and Drug Administration, but full harmonization is yet to come.
Health technology assessor and reimbursement criteria must be changed to address labeling issues for treatments identified from these new designs, which do not use large data sets or focus on superiority. For example, the European Medicines Agency proposed the use of pathogen-focused labeling, which indicates use for infections due to a genus or species of a bug (the Food and Drug Administration is still discussing this issue). Dr Rex proposed an anti-indication scheme in which one consider the use of a drug based on clinical data from other body sites and other pathogens, in cases where there are limited treatment options.
Good diagnostics increase trial efficiency. We need easily administered rapid results tests for use in selecting trial participants—even if they only select patients more likely to subsequently have a definitive culture. If the test increases the detection of a positive culture from 30% to 40%, the study size might go down by as much as 40%, thereby saving time and cost. Tests that incorporate direct-from-specimen methods, next-generation sequencing, and evaluation of host (patient) gene responses all hold promise.
We need a diverse, vibrant antibiotic pipeline. To achieve this, companies must be incentivized to increase their development efforts. This will require new funding approaches that take into consideration net present value (NPV) in the revenue model. A NPV>0 means that the investment has created some value, but review of the economics of antibiotics for 6 key indications showed the NPV of a new drug to be < $40 million, despite the fact that the value to society ranged from $500 million to $12 billion, based on value of days of work and life restored. This losing economic model is being positively changed by providing more global leadership and new public-private partnerships. Examples include the NIAID (Antibacterial Resistance Program) and BARDA (Biomedical Advanced Research and Development Authority) in the United States and the New Drugs for Bad Bugs (ND4BB) program in Europe. The ND4BB (sponsored by Europe's Innovative Medicines Initiative) is focused on improving our understanding of drug penetration into gram-negatives, using a data center to compile and analyze ND4BB-related information and ensuring best practices and communication of this information.
Streamlining antibacterial development also entails tackling economic issues and fostering responsible use of antibiotics. In particular, rewards to the innovator should not rest on only sales. Rewards might be derived like those obtained by an insurance program, where the US government acquires a fixed rate of new antibiotics from the developing company every year and an annual fee is guaranteed, whether the drug is used or not. The other approach is a refundable tax credit where the developing company receives either a tax credit or a payment of that amount for some percentage of qualified expenses. A systems approach to the problem is currently being developed that use these measures.
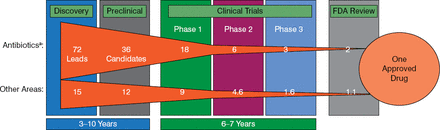
As it takes 10 to 20 years to make a new antibiotic, there is no time to lose (Figure 3).
Timeline for New Antibiotic Development
FDA, Food and Drug Administration.
aHit to phase 2 based on novel mechanism antibiotic discovery.
Adapted from Paul SM et al. Nat Rev Drug Discov. 2010.
Over the past 15 years, twice as many companies have stopped developing antibiotics than have entered the business. The lack of a diverse, vibrant pipeline of novel antibacterial agents is a global crisis that impairs our ability to treat life-threatening infections. The Infectious Diseases Society of America has set the challenge to develop 10 new systemic antibacterial drugs by 2020 using collaborative research and development, streamlined pathways, and new economic thinking that unleashes the power of private investment.
- © 2014 MD Conference Express®