Summary
Week 144 secondary end point results from a randomized, double-blind, double-dummy, active-controlled study involving 700 treatment-naïve HIV-1-infected patients have reaffirmed that cobicistat can be substituted for ritonavir in a drug regimen that seeks to boost the pharmacologic effect of atazanavir in combination with emtricitabine-tenofovir disoproxil fumarate.
- HIV & AIDS
- Infectious Disease Clinical Trials
- Infectious Disease
- HIV & AIDS
- Infectious Disease Clinical Trials
Week 144 secondary end point results from a randomized, double-blind, double-dummy, active-controlled study involving 700 treatment-naïve HIV-1-infected patients have reaffirmed that cobicistat (COBI) can be substituted for ritonavir (RTV) in a drug regimen that seeks to boost the pharmacologic effect of atazanavir (ATV) in combination with emtricitabine-tenofovir disoproxil fumarate (FTC-TDF). The noninferiority results were presented by Joel Gallant, MD, MPH, Southwest Care Center, Santa Fe, New Mexico, USA.
The enzyme inhibitor activity of RTV in combination with its inhibitory effect on the hepatic metabolism of many other drugs have made it a workhorse in highly active antiretroviral therapy (HAART) because it boosts the plasma levels of the other protease inhibitors, which allows reductions in their dose and frequency, while enhancing clinical activity.
COBI is a pharmacoenhancer that, in contrast to RTV, has no antiviral activity. Similar to RTV, it safely and effectively boosts the drug levels of darunavir, elvitegravir (EVG), and ATV by inhibiting their metabolism [German P et al. JAIDS. 2010; Gallant JE et al. J Infect Dis. 2013; Wohl DA et al. JAIDS. 2014]. The drug has been approved for use in HIV-1 infection by the European Union and the US Food and Drug Administration (FDA).
The study randomized 700 treatment-naïve HIV-1-infected patients (HIV-1 RNA ≥ 5000 copies/mL) 1:1 to receive ATV + COBI + FTC-TDF (n = 344) or ATV + RTV + FTC-TDF (n = 348). The primary end point of a decrease of HIV-1 RNA to < 50 copies/mL at 48 weeks was met, and it showed that COBI was noninferior to RTV in combination with ATV and FTC-TDF [Gallant JE et al. J Infect Dis. 2013]. The presently reported secondary end point was noninferiority at 144 weeks. Study arms were comparable at baseline concerning demographics, the prevalence of asymptomatic HIV infection, median HIV-1 RNA count, CD4 count, and estimated glomerular filtration rate (eGFR).
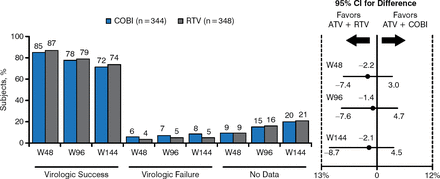
Similar to the 48-week results, the 144-week data show comparable HIV-1 RNA suppression and a low prevalence of virologic failure in both the COBI and RTV study treatment arms (Figure 1).
Virologic outcomes of cobicistat and ritonavir up to week 144
ATV, atazanavir; COBI, cobicistat; RTV, ritonavir.
Reproduced with permission from J Gallant, MD, MPH.
COBI and RTV were comparable in terms of the development of resistance by week 144 and the common adverse events associated with resistance. The drugs were also similar in the frequency of those adverse events that led to discontinued use of COBI or RTV (Table 1).
Reasons for Drug Discontinuation
The similarity extended to grade 3 and 4 laboratory abnormalities, predominantly hyperbilirubinemia (70% and 62% of abnormalities for COBI and RTV, respectively). Baseline to week 144 changes were similar for serum creatinine (0.13 for COBI, 0.07 for RTV) and eGFR (−15 for COBI, −8 for RTV). When creatinine and eGFR were tracked from weeks 4 to 144, eliminating the effects of COBI and RTV on tubular creatinine excretion, renal function was stable and comparable in both arms (serum creatinine: 0.04 for COBI, 0.02 for RTV; eGFR: −3.5 for COBI, −3.3 for RTV). There was no difference between the drugs concerning the ratio of total cholesterol to high-density lipoprotein.
Noninferiority of COBI versus RTV came with infrequent nucleotide and nucleoside reverse transcriptase inhibitor resistance and no protease inhibitor resistance. These data, and the recent approval of cobicistat by the FDA, will lead to coformulations with ATV and DRV.
- © 2014 MD Conference Express®