Summary
Among the strategies being explored to prevent infection with human immunodeficiency virus type 1 is ibalizumab, a humanized monoclonal antibody that binds to CD4 domain 2 and blocks the entry of HIV-1 into CD4-positive T cells. This article discusses the Safety Study of Ibalizumab Subcutaneous Injection in Healthy Volunteers (TMB-108) [NCT01292174] results of a study of the safety, tolerability, pharmacokinetics, and pharmacodynamics of subcutaneous injections of ibalizumab.
- HIV & AIDS
- Infectious Disease Clinical Trials
- HIV & AIDS
- Infectious Disease Clinical Trials
- Infectious Disease
Among the strategies being explored to prevent infection with human immunodeficiency virus type 1 (HIV-1) is ibalizumab, a humanized monoclonal antibody that binds to CD4 domain 2 and blocks the entry of HIV-1 into CD4-positive T cells. Steven Weinheimer, PhD, TaiMed Biologics, Irvine, California, USA, presented the results of a study of the safety, tolerability, pharmacokinetics (PK), and pharmacodynamics of subcutaneous (SC) injections of ibalizumab.
Safety Study of Ibalizumab Subcutaneous Injection in Healthy Volunteers (TMB-108) [NCT01292174] was a double-blind, placebo-controlled study that randomly assigned 8 participants at risk for acquiring HIV to each of 3 sequential escalating dose cohorts. Of the 24 participants enrolled, 89% were men; 65% were white; 23% were black; the mean age was 30 years; and the mean weight was 82 kg. Participants received 4 weekly SC injections of ibalizumab (120, 240, and 480 mg) or placebo. Follow-up continued for 26 weeks following dosing, and assessments included safety, PK, CD4 receptor occupancy, CD4 receptor density, and a CD4-dependent antibody response to hepatitis A virus (HAV) following challenge with HAV antigen at weeks 1 and 25.
There were no serious adverse events (AEs) and no discontinuations due to AEs. Treatment-related AEs occurred in 2 (28.6%) participants in the placebo group and 6 (31.6%) in the ibalizumab treatment groups (n = 4 of 7 in the 240-mg group; n = 2 of 6 in the 480-mg group). The most frequently reported treatment-emergent AEs were headache, upper respiratory tract infection, oropharyngeal pain and cough, and pruritus. No injection site reactions or anti-ibalizumab antibodies were observed, nor clinically significant changes in laboratory parameters, dose-response and temporal trends in vital signs, or physical examinations.
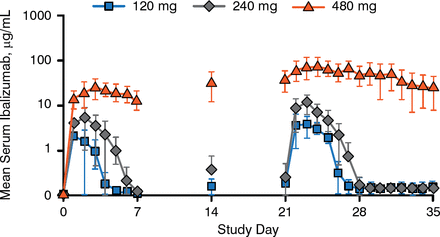
Daily serum ibalizumab concentrations after the first and fourth doses exhibited nonlinear PK consistent with target-mediated drug disposition, as shown in Figure 1. Higher first doses were associated with slower elimination, delayed absorption, and disproportionately higher systemic exposure.
Ibalizumab Mean Serum Concentrations
Reproduced with permission from S Weinheimer, PhD.
Maximum and trough serum concentrations and the area under the concentration-time curve increased with repeat doses. Results from 3 participants with body weights > 100 kg suggest that SC fat might delay the absorption of ibalizumab. All participants who received the highest dose had detectable levels of ibalizumab in their semen.
No participants had significant changes from baseline in CD4-positive T-cell counts. Anti-HAV antibodies were measured at weeks 5 and 29; antibody responses were detected in 100% of participants receiving placebo and in 53% and 94% receiving ibalizumab at these time points, respectively. The target for antiviral suppression is at least 85% CD4 receptor occupancy, which was achieved for 3 to 4 days after the first dose and for 6 to 7 days after the fourth dose of the 2 lower doses; nearly 100% receptor occupancy occurred for the entire dosing period for the highest dose.
Although the results suggest that SC ibalizumab may have the potential to prevent HIV-1 infection, the effects on antibody response to HAV antigen challenge require further study.
- © 2014 MD Conference Express®