Summary
The Single Tablet Regimen (STaR) head-to-head comparison of anti-HIV-1 formulations has established the noninferiority of rilpivirine/emtricitabine/tenofovir DF (RPV/FTC/TDF) versus efavirenz/emtricitabine/tenofovir DF (EFV/FTC/TDF) in HIV-1 virologic outcomes at weeks 48 and 96. The RPV/FTC/TDF combination was superior to EFV/FTC/TDF in terms of adverse effects and patient-reported outcomes.
- HIV & AIDS
- Infectious Disease Clinical Trials
- Infectious Disease
- HIV & AIDS
- Infectious Disease Clinical Trials
The Single Tablet Regimen (STaR) head-to-head comparison of anti-HIV-1 formulations has established the noninferiority of rilpivirine/emtricitabine/tenofovir DF (RPV/FTC/TDF) versus efavirenz/emtricitabine/tenofovir DF (EFV/FTC/TDF) in HIV-1 virologic outcomes at weeks 48 and 96. The RPV/FTC/TDF combination was superior to EFV/FTC/TDF in terms of adverse effects and patient-reported outcomes. The findings were presented by Calvin Cohen, MD, MSc, Community Research Initiative of New England, Boston, Massachusetts, USA.
The RPV/FTC/TDF combination for treatment of HIV-1 has been established as noninferior to EFV/FTC/TDF in patients naïve to antiretroviral therapy in 2 prior blinded placebo-controlled trials. However, the previous study regimens involved multiple pills and twice-daily dosing. STaR was a head-to-head comparison of single-tablet formulations of both drug combinations.
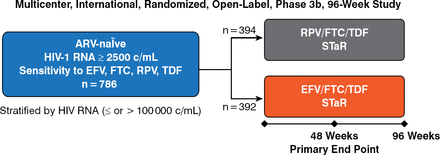
Antiretroviral-naïve patients (N = 786) with HIV-1 RNA ≥ 2500 copies/mL and sensitivity to the study drugs were randomized to the single-tablet formulations of RPV/FTC/TDF (n = 394) or EFV/FTC/TDF (n = 392) for 96 weeks (Figure 1).
Design of the STaR Trial
ARV, antiretroviral; EFV, efavirenz; FTC, emtricitabine; RPV, rilpivirine; STaR, single-tablet regimen; TDF, tenofovir.
Reproduced with permission from C Cohen, MD, MSc.
The primary end point was virologic success (reduction of HIV-1 RNA to < 50 copies/mL) at week 48. Secondary end points included proportion of subjects displaying virologic success at week 48; CD4 count at weeks 48 and 96; genotypic/phenotypic resistance at the time of virologic failure (rebound in HIV-1 RNA to ≥ 50 copies/mL); and subject-completed questionnaire-rated HIV symptoms, quality of life, and treatment satisfaction.
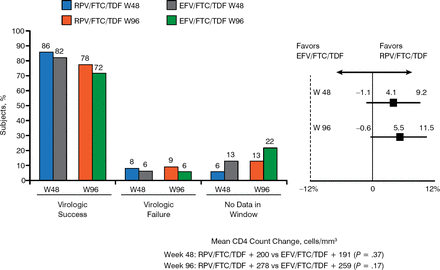
Baseline demographics, mean CD4 count, mean HIV-1 RNA, and proportion of coinfection with hepatitis B and/or C virus were similar between the groups. Figure 2 summarizes the virologic outcomes and CD4 change. The RPV/FTC/TDF combination produced slightly greater virologic success than the EFV/FTC/TDF combination at weeks 48 and 96. Virologic failure was low and similar for both formulations at weeks 48 and 96. The mean CD4 count increase at weeks 48 and 96 was greater but not statistically significant for the RPV/FTC/TDF formulation, with the overall change in CD4 counts favoring this formulation.
Virologic Outcomes and CD4 Change at Weeks 48 and 96
ARV, antiretroviral; EFV, efavirenz; FTC, emtricitabine; RPV, rilpivirine; TDF, tenofovir; W, week.
Reproduced with permission from C Cohen, MD, MSc.
Questionnaires completed at baseline and week 96 revealed that nervous system, psychiatric, gastrointestinal/related, and constitutional/other symptoms had developed less often and, when present, had resolved to a greater extent for those receiving the RPV/FTC/TDF formulation. The difference was statistically significant for pain/tingling in hands/feet (26% of RPV/FTC/TDF vs 40% of EFV/FTC/TDF), difficulty with sleep (37% vs 47%), depression (38% vs 49%), fatigue (38% vs 49%), fever (18% vs 27%), cough (17% vs 25%), hair loss (18% vs 27%), and sexual problems (26% vs 39%).
A battery of quality-of-life parameters were higher at week 96 compared to baseline in those receiving RPV/FTC/TDF than those receiving EFV/FTC/TDF, but the differences were not statistically significant. The mental health composite score (2.9 and 0.6 for RPV/FTC/TDF and EFV/FTC/TDF, respectively) favored the former drug combination, with significant differences in the RPV/FTC/TDF group between baseline and week 96 and as compared to the EFV/FTC/TDF group. The physical health composite score (0.7 and 1.7 for RPV/FTC/TDF and EFV/FTC/TDF, respectively) favored the latter group; the difference between the 2 groups was not significant.
The RPV/FTC/TDF combination was better tolerated, with fewer adverse effects, and was associated with significantly fewer adverse effect-related discontinuations (RPV/FTC/TDF, n = 10, 3%; EFV/FTC/TDF, n = 34, 9%; P < .001). The RPV/FTC/TDF combination was judged noninferior to EFV/FTC/TDF through week 96.
- © 2014 MD Conference Express®