Summary
This article provids a state-of-the-art update on antiretroviral therapy (ART) for HIV, including combinations to use as initial therapy, novel drugs in development, nucleoside-sparing treatment strategies, and reducing the drug burden.
- HIV & AIDS
- HIV & AIDS
- Infectious Disease
This session provided a state-of-the-art update on antiretroviral therapy (ART) for HIV, including combinations to use as initial therapy, novel drugs in development, nucleoside-sparing treatment strategies, and reducing the drug burden.
SELECTING INITIAL THERAPY
Ann Collier, MD, University of Washington and Harborview Medical Center, Seattle, Washington, USA, provided an overview of ART and discussed recommended agents. Factors to consider in selecting ART include regimen potency and durability, pretreatment HIV RNA level, resistance test results, potential adverse effects, comorbidities, drug-drug interactions, convenience, pregnancy potential, adherence potential, patient preference, cost, and availability.
For initial treatment regardless of viral load, the US Department of Health and Human Services [DHHS 2014] recommends selecting 3 drugs from 2 classes, not counting boosters (Table 1).
US DHHS Guidelines for Initial ART Regimens Regardless of Viral Load or CD4 Cell Count [DHHS 2014], With Yearly Costs
Efavirenz has been part of the first-line therapy for 15 years. It has high potency and viral suppression rates, prolonged efficacy, and simple daily dosing. Efavirenz also has a low genetic resistance barrier, however, and central nervous system adverse effects may persist. Direct comparisons show that efavirenz is inferior to the integrase strand transfer inhibitors (INSTIs), raltegravir [Lennox JL et al. J Acquir Immune Defic Syndr. 2010; Rockstroh JK et al. J Acquir Immune Defic Syndr. 2013] and dolutegravir [Walmsley SL et al. N Engl J Med. 2013].
According to Dr Collier, multiple excellent regimens are available for initial ART. No one regimen is optimal for all patients. Coformulated fixed-dose combinations are an important advance. Efavirenz-emtricitabine-tenofovir is no longer the best single option. There is much interest in integrase inhibitor-based regimens, but long-term data on elvitegravir and dolutegravir are lacking.
NEW AGENTS FOR ANTIRETROVIRAL THERAPY
Initial ART has been remarkably successful in some settings; however, as of 2010, only 68.4% of US patients receiving ART achieved viral suppression ≤ 200 copies/mL [CDC MMWR. 2011]. Joe Eron, MD, University of North Carolina, Chapel Hill, North Carolina, USA, discussed challenges to successful therapy and the development of new antiretroviral agents, including adherence to therapy, tolerability and toxicity, complex patients with comorbidities, and viral resistance.
Adherence can be improved by increasing convenience and developing long-acting agents. Four single-tablet regimens are currently available in the United States (dolutegravir-abacavir-lamivudine, efavirenz-tenofovir-emtricitabine, elvitegravir-cobicistat-tenofovir-emtricitabine, and rilpivirine-tenofovir-emtricitabine), and darunavir-cobicistat-tenofovir alafenamide (TAF)-emtricitabine is in development. Rilpivirine long-acting (LA) and cabotegravir LA are long-acting injectable agents designed as nanosuspensions with increased surface area and drug dissolution rate. The ongoing LATTE-2 study [NCT02120352] is evaluating the antiviral activity, tolerability, and safety of intramuscular cabotegravir LA plus rilpivirine LA in HIV-infected patients.
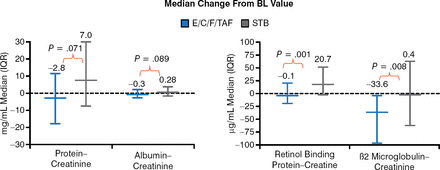
Tenofovir-emtricitabine is considered the best backbone with efavirenz, but it is associated with long-term renal toxicity and reduced bone density. Sax P et al. [J Acquir Immune Defic Syndr. 2014] found that elvitegravir-cobicistat-emtricitabine-TAF was associated with significantly less renal tubular proteinuria (Figure 1) and smaller changes in bone mineral density compared with elvitegravir-cobicistat-emtricitabine-tenofovir disoproxil fumarate (TDF).
Urine Tubular Protein Markers: 48-Week Analysis
BL, baseline; E/C/F/TAF, elvitegravir-cobicistat-emtricitabine-tenofovir alafenamide; IQR, interquartile range; STB, elvitegravir-cobicistat-emtricitabine-tenofovir disoproxil fumarate.
Reproduced from J Acquir Immune Defic Syndr, Sax PE et al. Tenofovir Alafenamide Vs Tenofovir Disoproxil Fumarate in Single Tablet Regimens for Initial HIV-1 Therapy: A Randomized Phase 2 Study, 2013;67:52–58, Copyright 2013, with permission from Lippincott Williams & Wilkins.
A phase 2b study showed that doravirine had comparable activity and fewer adverse effects compared with efavirenz [Morales-Ramirez et al. CROI 2014 Abstract 92LB].
Dolutegravir 50 mg BID has activity against many integrase-resistant variants. An in vitro study showed that TAF maintained activity against nucleoside reverse transcriptase inhibitor (NRTI)-resistant variants, whereas tenofovir level concentrations failed [Margot N et al. 2013]. A resistance analysis detected no resistance in 3 subjects treated with elvitegravir-cobicistat-emtricitabine-TAF, but resistance was detected in 2 of 3 subjects treated with elvitegravir-cobicistat-emtricitabine-TDF [Sax P et al. ICAAC 2013]. The attachment inhibitor prodrug, BMS-663068, has a unique resistance profile with no in vitro cross-resistance to other antiretroviral classes [Li Z et al. Antimicrob Agents Chemother. 2013]. A phase 2b study demonstrated the activity of BMS-663068 in treatment-experienced patients, but resistance developed in some patients [Lalezari J et al. CROI 2014 abstract 86].
Many new formulations are available, and more are planned that increase convenience, but cost generally is increased with these new agents. New agents in existing classes have been developed that may have decreased toxicity and improved tolerability. Few new agents have activity against resistant HIV variants, however.
REDUCING THE DRUG BURDEN
Triple ART is the standard of care for treatment of HIV infection. Pedro Cahn, Fundación Huésped, Buenos Aires, Argentina, explored the potential for reducing the drug burden. Potential advantages include reduced toxicity, improved adherence and quality of life, reduced drug-drug interactions, reduced cost, and the potential for longer-term success. Strategies for reducing drug burden include drug dose reduction, protease inhibitor (PI) monotherapy, class-sparing strategies, and dual therapy including lamivudine.
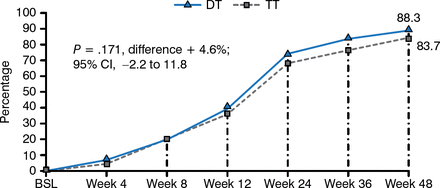
The Conference on Antiretroviral Drug Optimization reported that the efficacy of several antiretroviral drugs, including efavirenz, lopinavir-ritonavir (LPV/r), atazanavir, and darunavir, is maintained at doses that are lower than the approved dose [Crawford KW, Lancet Infect Dis. 2012]. The Global AntiRetroviral Design Encompassing Lopinavir/r and Lamivudine vs LPV/r based standard therapy [GARDEL; Cahn P et al. Lancet Infect Dis. 2014] study of 426 patients demonstrated that dual therapy with LPV/r plus lamivudine was noninferior to triple therapy after 48 weeks, regardless of baseline viral load. Virologic failure did not cause PI resistance development, preserving a wide range of drugs for second-line therapy.
The OLE study [NCT01471821]; Gattell JM et al. AIDS 2014] of patients with HIV < 50 copies/mL reported that switching to LPV/r plus lamivudine or emtricitabine was noninferior to continued LPV-RTV plus 2 NRTIs.
Dr Cahn concluded that well-designed noninferiority trials of dual therapy versus triple therapy with sufficient numbers of patients in new strategies and robust risk assessment are needed to change the treatment paradigm for HIV therapy. Criteria for identification of patients who are likely to benefit from new strategies and response durability assessment are needed.
NRTI-SPARING REGIMENS: HAS THE TIME FINALLY COME?
Mark Boyd, MD, The Kirby Institute, University of New South Wales, Australia, reviewed the evidence base for using NRTI-sparing regimens in ART-naïve and ART-experienced HIV-infected patients. Table 2 summarizes the results of studies in ART-naïve patients, whereas Table 3 summarizes the results of studies in ART-experienced patients.
The evidence for using NRTI-sparing regimens is strongest in ART-experienced patients. The NEAT 001/ANRS 143 study provides evidence for NRTI-sparing regimens as an alternative option in ART-naïve patients. Not all NRTI-sparing regimens are equal, however, as evidenced by the inferior efficacy of maraviroc paired with ritonavir-boosted darunavir instead of TDF-FTC fixed-dose combination NRTIs. Dr Boyd concluded that support is accumulating for NRTI sparing as a switch strategy for maintenance after conventional induction.
GARDEL Results: Viral Load < 50 Copies/mL at Week 48
BSL, baseline; DT, dual therapy; TT, triple therapy.
Reprinted from Lancet Infect Dis, 14, Cahn P et al, Dual Therapy With Lopinavir and Ritonavir Plus Lamivudine Versus Triple Therapy With Lopinavir and Ritonavir Plus Two Nucleoside Reverse Transcriptase Inhibitors in Antiretroviral-Therapy-Naïve Adults With HIV-1 Infection: 48 Week Results of the Randomised, Open Label, Non-Inferiority GARDEL Trial, 572–580, Copyright 2014, with permission from Elsevier Ltd.
NRTI-Sparing Regimens in ART-Naïve HIV-Infected Patients: Viral Suppression at 48 Weeks
NRTI-Sparing Regimens in ART-Experienced HIV-Infected Patients: Viral Suppression at 48 Weeks
- © 2014 MD Conference Express®