Summary
Part 1 of A Dose-Ranging Study to Compare MK-1439 Plus TRUVADA® Versus Efavirenz Plus TRUVADA® in Human Immunodeficiency Virus-1 Infected Participants (MK-1439-007) [NCT01632345] examined the safety, tolerability, pharmacokinetics, and efficacy of 4 doses of doravirine (25, 50, 100, and 200 mg once daily) versus efavirenz, both taken with emtricitabine-tenofovir. The Part 1 efficacy and safety results demonstrated that all 4 doses of doravirine had potent antiretroviral activity and fewer drug-related adverse events than efavirenz [Morales-Ramirez et al. CROI 2014].
- HIV & AIDS
- Infectious Disease Clinical Trials
- HIV & AIDS
- Infectious Disease
- Infectious Disease Clinical Trials
Commonly used nonnucleoside reverse transcriptase inhibitors (NNRTIs) are associated with suboptimal efficacy or safety profiles. Doravirine is a novel, next-generation NNRTI in development for the treatment of HIV-1 infection. It is dosed once daily and has a rapid onset with a median Tmax of 1 to 5 hours and an apparent terminal half-life of 11 to 19 hours. Doravirine is not expected to interact with proton pump inhibitors (PPIs) and can be dosed without regard to food intake.
Part 1 of A Dose-Ranging Study to Compare MK-1439 Plus TRUVADA® Versus Efavirenz Plus TRUVADA® in Human Immunodeficiency Virus (HIV)-1 Infected Participants (MK-1439-007) [NCT01632345] examined the safety, tolerability, pharmacokinetics (PK), and efficacy of 4 doses of doravirine (25, 50, 100, and 200 mg once daily) versus efavirenz, both taken with emtricitabine-tenofovir. The Part 1 efficacy and safety results demonstrated that all 4 doses of doravirine had potent antiretroviral activity and fewer drug-related adverse events (AEs) than efavirenz [Morales-Ramirez et al. CROI 2014]. In this report, Matthew L. Rizk, PhD, Merck and Company, Inc., Whitehouse Station, New Jersey, USA, presented exposure-response analysis results from Part 1 of this dose-ranging study.
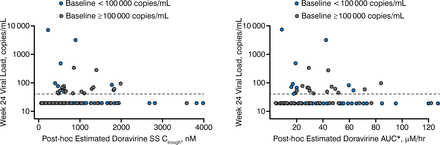
Sparse doravirine PK data, available from 167 of 208 enrolled patients, were pooled with densely sampled phase 1 PK data in a population PK model to obtain individual post-hoc estimates of steady-state PK parameters. Individual PK estimates and week 24 HIV-1 RNA (vRNA) were matched, and PK-pharmacodynamic (PD) trends were explored. Individual area under the curve (AUC) estimates were plotted against the predefined AE rate. Steady-state Ctrough distributions were compared to in vitro Ctrough targets for wild-type and various mutant HIV-1 strains.
The exposure-response analysis of efficacy showed that there was no trend between Ctrough or AUC and the proportion of patients with undetectable HIV-1 RNA at week 24 (Figure 1). No difference in the PK-PD relationship between high and low-viral-load patients was observed.
HIV RNA vs steady-state Ctrough and AUC0–24 hr by baseline HIV RNA
AUC, area under the curve; SS, steady-state.
Reproduced with permission from ML Rizk, PhD.
No apparent trend between AUC and the proportion of patients with central nervous system AEs, abnormal dreams, nausea, or diarrhea was observed. A logistic regression model found no significant relationship between AUC and nausea.
Comparison of steady-state Ctrough distributions and in vitro Ctrough targets for wild-type and mutant HIV-1 found that 25 mg through 200 mg doses of doravirine provided good coverage of wild-type virus, consistent with the clinical results (Table 1). The 100 mg dose provided better coverage (92.3%) of dual mutant strains [K103N/Y181C] compared with 50 mg, which provided 69.5% coverage.
Simulated Percentage of Patients With Higher-Than-Target Steady-State C24 hr for a Representative HIV-1 Patient Population Assuming Full Compliance
The safety and efficacy results showed that all tested doses of doravirine were efficacious and safe and had numerically higher response rates and fewer drug-related AEs than efavirenz. Although all 4 doravirine doses underwent exposure-response analysis, the study primarily focused on the 50 mg and 100 mg doses. The analysis confirmed that there is no relationship between doravirine plasma levels and both efficacy and AE rates. Based on these results and the superior coverage of dual mutant HIV-1 strains [K103N/Y181C], the 100 mg dose of doravirine was selected for Part 2 of the study (with additional enrollment at 100 mg doravirine vs efavirez) and for study in Phase 3.
- © 2014 MD Conference Express®