Summary
Results from a 48-week, open-label extension of A Trial Comparing GSK1349572 50mg Plus Abacavir/Lamivudine Once Daily to Atripla [SINGLE, NCT01263015], a phase 3, randomized, double-blind trial, have reaffirmed week 48 and week 96 results [Walmsley SL et al. N Engl J Med. 2013] of the superiority of once-daily dolutegravir 50 mg used with abacavir/lamivudine compared with efavirenz/tenofovir/emtricitabine in treatment-naïve patients with HIV-1.
- HIV & AIDS
- Infectious Disease Clinical Trials
- HIV & AIDS
- Infectious Disease Clinical Trials
- Infectious Disease
Results from a 48-week, open-label extension of A Trial Comparing GSK1349572 50mg Plus Abacavir/Lamivudine Once Daily to Atripla [SINGLE, NCT01263015], a phase 3, randomized, double-blind trial, have reaffirmed week 48 and week 96 results [Walmsley SL et al. N Engl J Med. 2013] of the superiority of once-daily dolutegravir (DTG) 50 mg used with abacavir/lamivudine (ABC/3TC) compared with efavirenz/tenofovir/emtricitabine (EFV/TDF/FTC) in treatment-naïve patients with HIV-1. The latest findings were reported by Keith Pappa, PharmD, GlaxoSmithKline, Research Triangle Park, North Carolina, USA.
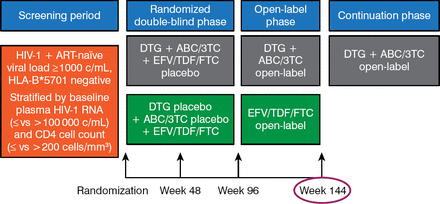
Prior to week 96, the study had been conducted in a double-blind fashion. From weeks 96 to 144, patients were free to remain on the therapy they had been randomized to with knowledge of the drug being used (Figure 1).
Design of the SINGLE Study
ABC/3TC, abacavir/lamivudine; ART, antiretroviral therapy; c/mL, copies per milliliter; DTG, dolutegravir; EFV/TDF/FTC, efavirenz/tenofovir/emtricitabine; HIV, human immunodeficiency virus; HLA-B, major histocompatibility complex, class 1, B57.1
Reproduced with permission from K Pappa, PharmD.
In the SINGLE study, 833 patients were randomized to receive daily DTG + ABC/3TC (n = 414) or EFV/TDF/FTC (n = 419). The primary end point at week 48 utilized the US Food and Drug Administration snapshot analysis, which is the proportion of patients with a last available HIV-1 RNA within the visit of < 50 copies/mL. Secondary end points included the proportion of patients with decreased HIV-1 RNA to < 50 copies/mL at week 144, the change from baseline in the CD4+ T cell count, long-term safety, immune response, health outcomes, and emergence of viral resistance. The study arms were comparable at baseline in terms of age, sex, race (African American), proportion of US Centers for Disease Control and Prevention class C, median HIV-1 RNA count, and median CD4+ count.
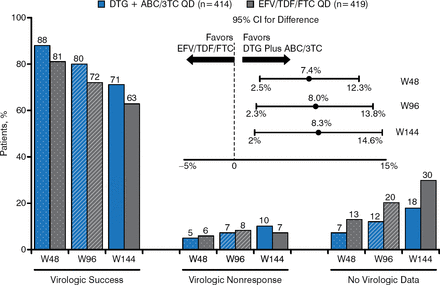
At week 144, the proportion of patients having HIV-1 RNA viral load < 50 copies/mL was significantly higher in the DTG + ABC/3TC arm than in the EFV/TDF/FTC arm (71% versus 63%; adjusted treatment difference between groups: +8.3%; 95% CI, 2.0 to 14.6; P = .010). The change in CD4 count from baseline to week 144 was significantly greater in the DTG + ABC/3TC arm than in the EFV/TDF/FTC arm (adjusted mean ± SE, 378.5 ± 11.0 versus 331.6 ± 11.6; 95% CI, 15.6 to 78.1; P = .003).
Figure 2 presents snapshot outcomes by analysis time points with virologic success significantly greater for the DTG regimen at each time point. The forest plot data demonstrate superiority at each time point.
HIV-1 RNA Results at Week 144
ABC/3TC, abacavir/lamivudine; DTG, dolutegravir; EFV/TDF/FTC, efavirenz/tenofovir/emtricitabine; HIV, human immunodeficiency virus; W48, week 48; W96, week 96; W144, week 144.
Reproduced with permission from K Pappa, PharmD.
Protocol-defined virologic failure (PDVF) was defined as confirmed HIV-1 RNA ≥ 50 copies/mL at or after week 24. Among patients taking DTG + ABC/3TC, PDVF was evident in 39 patients (9%), none due to identifiable mutations. In the EFV/TDF/FTC group, PDVF was evident in 33 patients (8%), 13 of which had identifiable non-nucleoside (in 6 patients) and nucleoside (in 1 patient) reverse transcriptase inhibitor mutations. At week 144, the difference between the DTG + ABC/3TC arm and the EFV/TDF/FTC arm for patients entering the trial with ≤ 100 000 copies/mL HIV-1 RNA (73% versus 64%, respectively), > 100 000 copies/mL HIV-1 RNA (69% versus 61%, respectively), ≤ 200 CD4 cells/μL (60% versus 56%, respectively), and > 200 CD4 cells/μL (73% versus 64%, respectively) was consistent with the overall treatment difference observed for the population studied.
The drug-related adverse effects profile evident at week 96 showed little change to week 144 (Table 1).
Change in Common Drug-Related Adverse Events, Week 96 to Week 144
The number and proportion of serious adverse events through week 144 were similar in each arm. However, the number of serious drug-related adverse events was lower in the DTG + ABC/3TC group (n = 2 [< 1%]) compared with the EFV/TDF/FTC group (n = 9 [2%]). The same trend was evident for adverse events that led to withdrawal through week 144 (n = 16 [4%] versus n = 58 [14%]). As reported at weeks 48 and 96, the DTG group had small, nonclinically meaningful, nonprogressive changes in serum creatinine, due to known inhibition of creatinine tubular secretion by DTG.
The latest findings from this open-label extension of the SINGLE study reaffirm the earlier SINGLE findings that the DTG + ABC/3TC regimen is superior to EFV/TDF/FTC in treatment-naïve patients with HIV-1.
- © 2014 MD Conference Express®