Summary
Over the past decades, guidelines have evolved toward a much more aggressive approach to lowering lipids. Despite this, large numbers of cardiovascular events continue to occur. This clearly demonstrates that lowering low density lipoprotein is not sufficient, and that additional interventions are needed. Developing therapies which raise high-density lipoprotein (HDL) have become promising targets. Many researchers believe that HDL interacts with the vascular wall to promote removal of cholesterol.
- Lipid Disorders
Daniel J. Rader, MD, of the University of Pennsylvania School of Medicine was chosen to give the American College of Cardiology 2007 Bishop Lecture. The topic of HDL research was a timely one, given the results of several late breaking clinical trials.
Over the past decades, guidelines have evolved toward a much more aggressive approach to lowering lipids. Despite this, large numbers of cardiovascular events continue to occur. This clearly demonstrates that lowering low density lipoprotein (LDL) is not sufficient, and that additional interventions are needed.
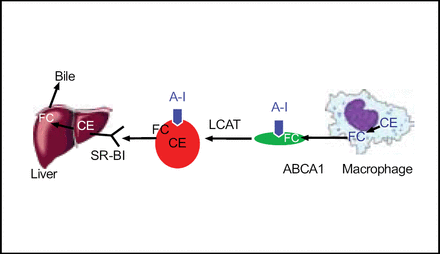
Developing therapies which raise high-density lipoprotein (HDL) have become promising targets. Many researchers believe that HDL interacts with the vascular wall to promote removal of cholesterol (Figure 1).
HDL Metabolism and Reverse Cholesterol Transport.
HDL may also have anti-inflammatory properties that benefit the vascular system. This belief that HDL plays a protective role in atherosclerosis stems mostly from a strong inverse epidemiologic association between high HDL and low incidence of cardiovascular disease (CVD). Observations from longitudinal studies such as the Framingham study found that an increase in HDL was associated with significant decreases in CVD.
Preclinical animal models have also demonstrated that hepatic over-expression of apolipoprotein A1 (Apo-A1) halted progression and sometimes led to regression of atherosclerosis in mice. This suggests a causal relationship between HDL, Apo-A1, and atherosclerosis. Clinical trials to date, however, have not clearly upheld this idea. “Many of us look to the time when we will have the tools to actually prove definitively, or disprove, that the HDL hypothesis really is true, that HDL is causally related to atherosclerosis in humans”, said Dr. Rader.
Molecule vs Mechanism
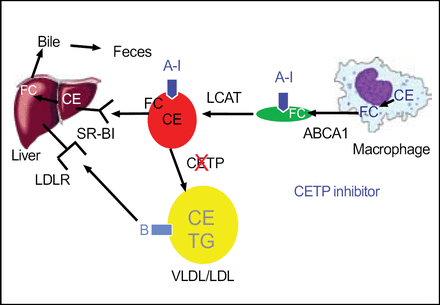
Cholesterol ester transfer protein (CETP) plays a major role in the transfer of cholesterol esters between lipoproteins. Patients who have a CETP deficiency have noticeably higher levels of HDL and Apo-A1. It is logical therefore that inhibiting CETP is a potential strategy for increasing HDL (Figure 2).
CETP Inhibition as a Novel Strategy to Raise HDL-C.
Torcetrapib, a CETP inhibitor, looked very promising as an agent to increase HDL and in the ILLUSTRATE trial. Treatment with torcetrapib resulted in unprecedented increases in HDL. Torcetrapib research was discontinued, however, because of the excess mortality observed in ILLUSTRATE, which also showed significant increases in blood pressure. Although torcetrapib succeeded in its goal of generating large increases in HDL, there was no significant impact of the compound on atherosclerosis.
Several unanswered questions regarding CETP inhibition remain. It is natural to wonder how much of the unfavorable safety data were related to the compound itself, how much was related to CETP-inhibition, and whether the blood pressure increases were linked to a vascular-toxic effect. CETP-deficient patients do not appear to have hypertension. Unlike torcetrapib, infusions of another CETP-inhibitor (MK0859) did not cause blood pressure increases in mice. “These data…really allow us to say convincingly that the blood pressure effect is a molecule-specific effect”, said Dr. Rader. “Is CETP-inhibition still viable as a therapeutic strategy? I think so. I think that we have data that suggest that this might be an approach, that with a clean compound that doesn't have some of the blood pressure and potentially vascular-toxic issues, there is still potentially some opportunity to demonstrate the beneficial effects.”
HDL Research Post-Torcetrapib
Several areas hold promise in the field of HDL research, but overall Dr. Rader believes that the emphasis will be on improving HDL and reverse cholesterol transport (RCT) function. “I think it's quite clear, and I think the one thing the torcetrapib experience really tells us, is that increasing HDL levels is neither adequate nor necessary for predicting the cardiovascular benefit of an HDL-targeted therapeutic approach”, he noted. Potential therapeutic pathways should instead focus on the HDL function, and a variety of promising options exist:
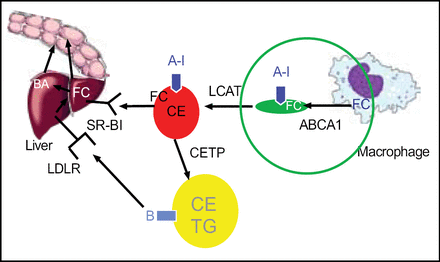
Increasing acceptor concentrations such apoA-I infusion or upregulation (Figure 3).
Increasing cholesterol efflux pathways such as ATP-binding cassette (ABC)A1 and ABCG1 to enhance cholesterol transport pathways.
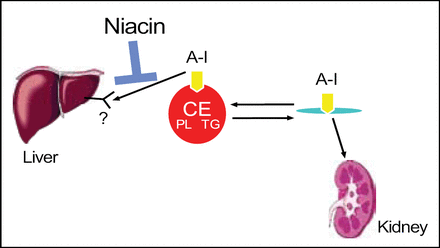
Niacin receptor agonists that might increase HDL. Niacin may block uptake of Apo-A1, and this mechanism needs to be further defined (Figure 4).
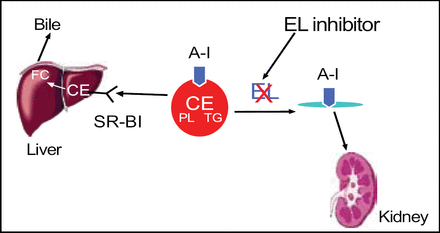
Endothelial lipase inhibitors might increase HDL by reducing its metabolism (Figure 5). Studies in patients with metabolic syndrome have shown an up-regulation of endothelial lipase, resulting in lower levels of HDL. “We think endothelial lipase is one of the missing links between obesity and low HDL that we see so commonly,” said Dr. Rader.
The liver × receptor (LXR) modulates the ABCA1 and ABCG1 pathways. LXR agonists are attractive candidates as they would activate the cholesterol efflux pathways. In mice, LXR agonist GW3965 resulted in increased cholesterol efflux, and in other mice models LXR agonists have caused significant decreases in atherosclerosis.
Compounds such as Apo-A1 mimetics that enhance anti-inflammatory HDL properties.
Increasing Lipid-Poor apoA-1 as an Acceptor for Cholesterol Efflux via ABCA1.
Niacin May Reduce Hepatic Uptake of HDL apoA-1.
Endothelial Lipase: Target for Pharmacologic Inhibition to Raise HDL?
“As we turn more to function and away from HDL cholesterol as a key biomarker of efficacy, we're going to desperately need better measures and biomarkers of HDL function and reverse cholesterol transport in humans. I think for the field this is critically important,” concluded Dr. Rader.
- © 2007 MD Conference Express