Summary
Prophylactic cranial irradiation in the treatment of extensive-stage small cell lung cancer has proven value in reducing symptomatic brain metastases and in improving overall survival at 1 year. However, persistent intrathoracic disease occurs in 76% of such patients and intrathoracic disease progresses in almost 90% of this group. This article discusses data from the Chest Radiotherapy Extensive Stage Trial [CREST; NTR1527] that evaluated the use of thoracic radiotherapy in addition to PCI for treatment of ES-SCLC to prevent occurrence of thoracic disease and halt its progression.
- Cancer
- Radiation Therapy
- Oncology Clinical Trials
- Radiology
- Respiratory Cancers
- Cancer
- Radiation Therapy
- Oncology
- Oncology Clinical Trials
- Radiology
- Respiratory Cancers
Prophylactic cranial irradiation (PCI) in the treatment of extensive-stage small cell lung cancer (ES-SCLC) has proven value in reducing symptomatic brain metastases and in improving overall survival (OS) at 1 year [Slotman B et al. N Engl J Med. 2007]. However, persistent intrathoracic disease occurs in 76% of such patients and intrathoracic disease progresses in almost 90% of this group. Ben J. Slotman, MD, PhD, Vrije Universiteit Medical Center, Amsterdam, The Netherlands, presented data from the Chest Radiotherapy Extensive Stage Trial [CREST; NTR1527] that evaluated the use of thoracic radiotherapy (TRT) in addition to PCI for treatment of ES-SCLC to prevent occurrence of thoracic disease and halt its progression.
Eligibility requirements for CREST were patients aged ≥ 18 years with ES-SCLS disease, who had a World Health Organization (WHO) performance status score of 0 to 2 and had shown either a complete response (CR), a partial response (PR), or a “good response” to 4 to 6 platinum-based chemotherapy treatments. Exclusions included any brain, leptomeningeal, or pleural metastases, and any previous brain radiotherapy or TRT. Patients were randomized and stratified by institute and presence or absence of intrathoracic disease, and then received PCI 2 to 7 weeks after chemotherapy and either TRT (10 fractions of 3 Gy) or no TRT. The primary end point of the study was OS, with secondary end points of progression-free survival (PFS), local control, failure pattern, and toxicity. The study was designed at 80% power to detect a hazard ratio of 0.76 for OS at 1 year (2-sided, with 5% significance). Accounting for a 5% dropout rate between randomization and start of treatment, 483 patients needed to be randomized. Accrual was successful, with 498 patients enrolled in 42 centers primarily in The Netherlands and the United Kingdom. The median patient age was 63 years, and 55% of participants were men and 45% were women. Over one-half of patients had a WHO performance status score of 1, whereas another one-third had WHO 0 and 10% had WHO 2 scores. In terms of their response to chemotherapy, just over 70% of patients had PR, one-fourth showed “good” response, and just 5% had a CR. As seen in other studies, nearly 90% of such patients with ES-SCLC treated with chemotherapy had persistent intrathoracic disease.
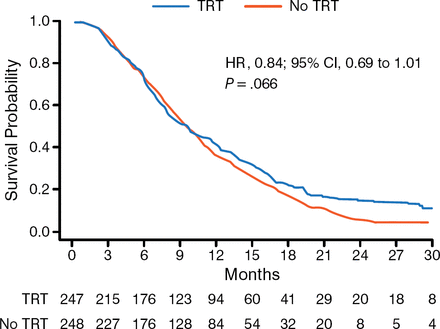
The primary end point was not met for OS because the hazard ratio did not meet criteria for statistical significance (HR, 0.84; 95% CI, 0.69 to 1.01; P = .066). However, in reviewing the OS curves, Dr Slotman said that the curves did begin to diverge in a statistically significant way at about 9 to 12 months in favor of TRT, and that the survival at 24 months was “highly statistically significant” (P = .004). Intrathoracic progression data showed strong statistical significance, with 43.7% of patients progressing who had received TRT compared with 79.8% for the control group (P < .001). Treatment was well tolerated, with all toxicities (cough, dysphagia, dyspnea, esophagitis, fatigue, insomnia, nausea/vomiting, and headache) with no difference between the arms and restricted to grade 3 on the Common Terminology Criteria for Adverse Events v3 scale. Dr Slotman concluded that TRT administered in addition to PCI for patients with ES-SCLC improved OS and PFS, and that TRT should be offered in addition to PCI to patients with a response to initial chemotherapy.
Overall Survival in CREST
CREST, Chest Radiotherapy Extensive Stage Trial; TRT, thoracic radiotherapy.
Reproduced with permission from BJ Slotman, MD, PhD.
- © 2014 MD Conference Express®