Summary
This article summarizes several presentations which discussed recent publications that covered important advances in vaccinology.
- Vaccinations
- Screening & Prevention
The following is a summary of several presentations which discussed recent publications that covered important advances in vaccinology.
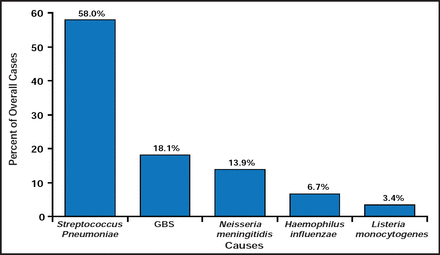
Kathryn M. Edwards, MD, Vanderbilt University, Nashville, Tennessee, USA, opened the session with a discussion of a publication that analyzed bacterial meningitis trends in the United States between 1998 and 2007 [Thigpen MC et al. N Engl J Med 2011]. Using data from eight surveillance areas, consisting of approximately 17.4 million persons, the authors identified 3188 cases of meningitis. During the study period, there was a 31% decrease in the incidence of meningitis and an increase in the median age of patients from 30.3 to 41.9 years. There was no change in case fatality rate (15.7% to 14.3%) or in the rate of disease in children aged <2 months. Dr. Edwards noted the marked reduction, by as much as 50% to 60%, in cases among children aged 2 to 10 years, which she attributed to the introduction of the conjugate pneumococcal vaccine in 2000. The study showed a much greater change in children than in adults, which raises a question as to whether herd immunity is going to be good enough to allow children to be immunized, without having adults immunized. In concluding, Dr. Edwards noted that while the rates of meningitis have decreased since 1998, largely due to effective immunization programs among children, the burden of disease is now being borne by adults, for whom new vaccine approaches are needed. In addition, there remains an enormous burden of Group B Streptococcus (GBS) disease in children aged <2 months that still needs to be addressed (Figure 1).
Proportion of the 1670 Cases of Bacterial Meningitis Reported in 2003 to 2007 Caused by Each Pathogen, According to Age Group.
Reproduced with permission from KM Edwards, MD.
Dr. Edwards also discussed an animal study that provided a new method of identifying protective antigens of Staphylococcus aureus [Kim HK et al. FASEB J 2011]. Staphylococcal sepsis and skin and soft tissue infections are major problems. Antibiotic resistance is increasing, there are no vaccines to prevent staphylococcal infections, and staphylococcal infection does not confer immunity from repeat infection. This study hoped to address two questions: 1) whether a live, attenuated vaccine for S. aureus could be developed; 2) whether this information could be used to contribute to our understanding of the pathogenesis of staphylococcal infections.
An early step in this study was the development of a renal abscess model, in which mice were injected with various strains of Staph. Those strains that did not produce an abscess were then injected into another cohort of mice that were treated with antibiotics on Day 19, rechallenged with virulent Staph at Day 26, and sacrificed on Day 30, after which their kidneys were assessed for bacterial load and the presence of abscesses. “There were three interesting findings,” said Dr. Edwards. “Mice that were not vaccinated had lots of bacteria and abscesses when they were rechallenged with wild-type Staph. After vaccination and then rechallenge with wild-type Staph, there was no change in the load of the Staph nor in the number of abscesses; however, inoculation with srtA variants (and, to a lesser extent, saeR variants) led to a diminution in the number of organisms and in the number of abscesses, indicating that it confers some protection against rechallenge with virulent Staph.”
In the next step, the animals were immunized using the isolated antigens that were identified in the prior step (Combo 1: ClFA, fibronectin-binding protein, SdrD; or Combo 2: ClfA, fibronectin-binding protein, SdrD, and SpA) or a mock injection and then rechallenged with virulent Staph. When the bacteria in the renal tissues were counted at Day 4, animals that received the mock injection had about 4.5 logs; for Combo 1, it was 2.5 logs, and for Combo 2, there were about 1.5 logs. At Day 18, there was a further diminution in colony-forming units in Combo 1. Essentially no organisms were seen with Combo 2. In the final step, the animals were given active immunization with Combo 1 or 2 and then given lethal doses of Staph. Four days postchallenge, all animals that received mock injections had died; 30% of the remaining animals survived to Day 18, with the survival rate of Combo 2 being significantly better than with Combo 1, suggesting that these antigens should be further studied. Dr. Edwards concluded by stating that the attenuated Staph strains induced protection and that combinations of Staph antigens resulted in nearly complete protection against lethal challenge in mice.
Jacek Mrucowicz, MD, Polish Institute for Evidence Based Medicine, Krakow, Poland, presented data from a Canadian study of the AS03-adjuvanted pandemic H1N1 vaccine [Skowronski DM et al. Br Med J 2011]. The study group comprised 209 confirmed flu patients from over 500 community-based clinics. Participants were mostly (>80%) children and adults aged <50 years who received a single vaccination using the AS03 vaccine at least 14 days prior to their flu diagnosis. Data were collected during two periods: November 8 to December 5, 2009 (primary) and November 1 to December 31 2009 (secondary). The investigators concluded that the AS03-adjuvanted vaccine that was used was highly effective (adjusted vaccine effectiveness 93% [95% CI, 69% to 98%]) in preventing medically attended, laboratory-confirmed pandemic H1N1 illness. Similar positive results for the 2009–2010 pandemic and seasonal influenza vaccines were seen in the European I-MOVE trial [Valenciano M et al. PLOS Med 2011]. Prof. Mrucowicz concluded by saying that although both studies found the adjuvanted H1N1 flu vaccines to be effective, as with all observational trials, there remains room for uncertainty.
Carol J. Baker, MD, Baylor College of Medicine, Houston, Texas, USA, discussed the results of a study from the United Kingdom that assessed the kinetics of immune responses to nasal challenge with meningococcal polysaccharide 1 year after serogroup glycoconjugate vaccination [Wing JB et al. Clin Infect Dis 2011]. The study objective was to measure the persistence of antibodies to serogroup C N. meningitidis 1 year postimmunization with meningitis C conjugate vaccine (CV) and to investigate the kinetics of systemic and mucosal immune responses to intranasal challenge with meningococcal group C polysaccharide vaccine (PV). The study enrolled 116 vaccine-naïve young adults (median age 23 years) who received meningococcal C CV. Eighty-nine participants (77%) consented to have meningococcal C PV inoculated into each nostril at 1 year. All subjects had protective (≥8) serum bactericidal antibody titers (SBA) 28 days after the initial CV vaccination. One patient failed to respond to immunization when a 1:128 titer was used for response. After 12 months, 12.3% had SBA titers <8 (20.2% using a titer of 1:128). In the 12% to 20% of healthy adults who failed to retain protective serum bactericidal antibody (SBA) titers 1 year after meningitis C CV, immunological memory was unable to create a protective systemic or mucosal bactericidal antibodies until 7 days postchallenge. It is possible that the speed of this response is too slow to protect from natural meningococcal infection.
In the United States, we have had an adolescent vaccine program with a quadravalent conjugate vaccine (MenACWYD) for several years. Protective SBA titers in 50% of the adolescents who received the vaccine have fallen to nonprotective levels 5 years after vaccination. The data that were presented by Wing et al. support the recent ACIP recommendation for a booster dose of MenACWYD vaccine at age 16 years for individuals who were first vaccinated before the age of 15 years. The booster should provide protection through age 20 years, when the incidence of invasive meningococcal disease falls.
- © 2011 MD Conference Express