Summary
Aromatase inhibitors are important agents used for the adjuvant treatment of early hormone receptor-positive breast cancer in postmenopausal women. Studies in early stage breast cancer are ongoing to determine if the cellular proliferation biomarker Ki67 predicts patient response to therapy. Genetic analysis of biopsy specimens may help identify resistance mechanisms.
- hormone receptor-positive
- postmenopausal women
- breast cancer
- clinical research
- biomarker analysis
- anastrozole
- tamoxifen
- Arimidex, Tamoxifen Alone or in Combination
- ATAC
- NCT00894030
- Immediate Preoperative “Arimidex” (anastrozole), Tamoxifen, or Arimidex Combined with Tamoxifen
- IMPACT
- Trial of Perioperative Endocrine Therapy – Individualising Care
- POETIC
- NCT02338310
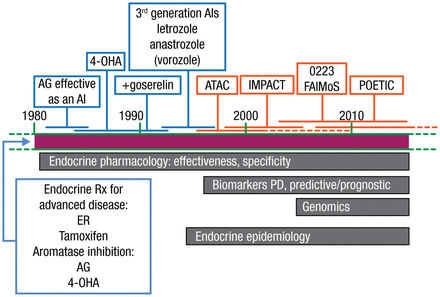
Mitchell Dowsett, PhD, Royal Marsden Hospital, London, United Kingdom, gave a brief history of the development of aromatase inhibitors (AIs) and an overview of ongoing research seeking to further elucidate the role of hormones in breast cancer (BC). Developed in the 1990s, AIs are approved for the adjuvant treatment of early hormone receptor-positive BC in postmenopausal women. A timeline of AI development according to Dr Dowsett’s lab is illustrated in Figure 1.
Timeline of Aromatase Inhibitor Development
4-OHA, 4-hydroxyandrostenedione; AG, aminoglutethimide; AI, aromatase inhibitor; ER, estradiol; PD, pharmacodynamics.
Reproduced with permission from M Dowsett, PhD.
The availability of pharmacodynamic biomarkers helped to accelerate the development of AIs. Plasma estradiol assays with high sensitivity and specificity were particularly useful in letrozole and anastrozole research. According to Dr Dowsett, biomarker analyses of these agents demonstrated that the extreme suppression of estrogen levels would lead to clinical responses that no true phase 2 trials had been studied. It is estimated that this reduced the development time by 12 to 18 months.
In the ATAC trial [Baum M et al. Lancet. 2002], anastrozole was compared with tamoxifen and a combination of anastrozole plus tamoxifen as adjuvant therapy in > 9000 patients with estrogen receptor (ER)-positive early BC. At 3 years, there was a significant improvement in the anastrozole group compared with the tamoxifen group (HR, 0.83; 95% CI, 0.71 to 0.96; P = .013). This benefit was shown to persist at 10-year follow up [Cuzick et al. Lancet Oncol. 2010].
The IMPACT trial [Dowsett M et al. Clin Cancer Res. 2005] was similar to the ATAC protocol but biopsy specimens were taken before and at 2 and 12 weeks after treatment initiation. The change in the cellular proliferation marker Ki67 was significantly greater with AI treatment vs tamoxifen (P = .004 at 2 weeks and P < .001 at 12 weeks) and suggested that Ki67 may correlate with patient outcomes. To further investigate whether the pretreatment or 2-week Ki67 levels predict recurrence-free survival, the POETIC trial [Dowsett M et al. J Natl Cancer Inst Monogr. 2011] has randomized nearly 4500 patients with early stage ER-positive BC. Two-thirds of the patients were randomized to AI therapy before surgery, while the other patients received standard hormone therapy. Biopsies and Ki67 measurements were conducted at diagnosis and 2 weeks later at surgery.
Other research has shown that plasma levels of estradiol are correlated with the expression of estrogen-regulated genes in postmenopausal ER-positive BC [Dunbier AK et al. J Clin Oncol 2010]. To date, 3300 core biopsy pre- and post treatment samples have been obtained in the POETIC study. Whole genome expression was suppressed in 926 and increased in 401. Dr Dowsett commented that this provided the opportunity to try to identify patient-specific mechanisms of resistance. To see if the menstrual cycle can be utilized as a test of hormone sensitivity in premenopausal women, the MenCER study was designed and has completed recruitment. Seventy paired biopsies from participants have been obtained and analysis is ongoing.
- © 2014 SAGE Publications